Translate this page into:
Severe Rhabdomyolysis Due to Presumed Drug Interactions of Dapagliflozin with Rosuvastatin: A Case Report
Corresponding author: Anaghashree Udayashankar, Department of Nephrology and Renal Transplantation, Aster Whitefield, Bengaluru, India. E-mail: anaghashree1702@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Udayashankar A, Mukherjee T, George K, KrishneGowda K. Severe Rhabdomyolysis Due to Presumed Drug Interactions of Dapagliflozin with Rosuvastatin: A Case Report. Indian J Nephrol. doi: 10.25259/IJN_25_2024
Abstract
Sodium-glucose-cotransporter-2 inhibitors (SGLT2i) and statins are increasingly used for reduction of cardiovascular mortality in type 2 diabetics. Few case studies reported an enhanced risk of rhabdomyolysis with this combination. A 57-year-old man with normal renal functions, developed fatigue and oliguria within three days of dapagliflozin addition to his preexistent rosuvastatin therapy. Investigations revealed severe acute kidney injury (AKI) with elevated serum creatine-phosphokinase (CPK) and myoglobinuria. Renal biopsy depicted severe acute tubular necrosis with interstitial nephritis and ropy myoglobin casts, which confirmed the diagnosis of rhabdomyolysis. Rosuvastatin and dapagliflozin were discontinued. Hemodialysis and oral steroids were prescribed. The AKI recovered within few weeks. Rosuvastatin was rechallenged after two months and his renal functions and CPK levels remained normal. This case demonstrates the incidence of severe rhabdomyolysis when an SGLT2i was added to an existing statin, emphasizing the importance of identifying drug-drug interactions and potential for myotoxicity.
Keywords
Rhabdomyolysis
Dapagliflozin
Rosuvastatin
Acute kidney injury
Drug-drug interactions
Introduction
Statin-associated rhabdomyolysis (SAR) is well documented.1 Coadministration of a sodium-glucose cotransporter-2 inhibitor (SGLT2i) can increase the risk of myotoxicity.2 We report the case of a man with severe acute kidney injury (AKI) that resolved when incriminatory drugs were stopped.
Case Report
A 57-year-old man with type 2 diabetes and coronary artery disease developed a three-day history of fatigue, exertional dyspnoea, pedal edema, and oliguria. There was a fever 10 days back that subsided on its own. Medication history included ticagrelor, rosuvastatin, and beta blockers. During his routine check-up three days before symptom onset, his serum creatinine level was 0.9 mg/dL. During this visit, 10 mg of dapagliflozin was prescribed.
Clinical examination revealed a blood pressure of 200/100 mm Hg, pedal edema, and bilateral basal crepitations on lung auscultation.
Evaluation revealed severe AKI with hyperkalemia, severe metabolic acidosis, bland urine sediment [Table 1], and normal abdominal ultrasound. Hemodialysis was initiated and the possible etiologies of AKI considered were tropical fever or a drug-related nephrotoxicity. The only identifiable trigger between the normal report of creatinine and the current episode was the introduction of dapagliflozin. Additional investigations revealed elevated serum creatine phosphokinase (CPK), aspartate aminotransferase, alanine aminotransferase and myoglobinuria [Table 1].
| Parameters | Day 0 | Day 2 | Day 7 | Day 16 | Day 21 | Day 28 | Day 90 |
|---|---|---|---|---|---|---|---|
| Hemodialysis (HD) | HD initiated | HD thrice weekly | HD | Last HD | |||
| Blood urea (mg/dl) | 117 | 124 | 89 | 143 | 74.9 | 29 | 27.82 |
| S. Creatinine (mg/dl) | 11.4 | 9.5 | 7.6 | 6.6 | 3.6 | 2.3 | 1.4 |
| Potassium (mEq/L) | 7.1 | 4.2 | 3.6 | 4.2 | 3.9 | 4.7 | 5 |
| Uric acid (mg/dl) | 7.3 | 6.4 | 5 | 5 | 5.3 | 4.1 | 5.4 |
| Hemoglobin (g/dl) | 10 | 7.2 | 8.6 | 10.4 | |||
| White cell count (/uL) | 11,810 | 7390 | 10,790 | 6560 | |||
| Platelets (Lakh/uL) | 2.25 | 1.76 | 5.89 | 3.34 | |||
| CPK (IU/ml) | 10,980 | 8736 | 213 | 203 | |||
| AST (IU/ml) | 268 | 141 | 14 | ||||
| ALT (IU/ml) | 500 | 352 | 20 | ||||
| Miscellaneous |
LDH 496 IU/ml HIV, HbSAg, HCV: Not reactive Calcium 7.5 mg/dl Phosphorus 6.4 mg/dl Magnesium 1.4 mg/dl Total protein: 6 Albumin: 3.2 |
Dengue, Malaria parasite, Leptospira Rickettsia: Negative Urine myoglobin >1200 ng/ml |
Left Renal Biopsy done |
Urine Protein: Nil RBC, WBC: Nil Urine ACR: 8 mg/g |
mEq/l: milliequivalents per liter mg/dl (milligrams/deciliter); g/dl: grams/deciliter; uL: microliter; IU/ml: international units/milliliter; LDH: lactate dehydrogenase; CPK: creatinine phosphokinase; AST: aspartate aminotransferase; ALT: alanine aminotransferase; HIV: human immunodeficiency virus; HbSAg: hepatitis B surface antigen; HCV: hepatitis C virus; ACR: albumin creatinine ratio.
A diagnostic renal biopsy revealed severe acute tubular necrosis with ropy granular casts, which stained positive for myoglobin and moderate acute interstitial nephritis (AIN) without diabetic nephropathy features, which confirmed rhabdomyolysis [Figures 1 and 2].
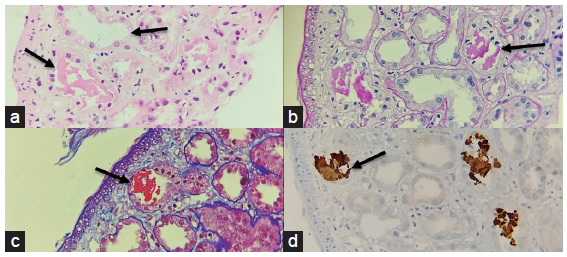
- A panel of photomicrographs (400x) of the renal biopsy with black arrows depicting acute tubular injury with ropy and globular casts which are eosinophilic (a) H/E stain, (b) weakly PAS positive (PAS stain) (c) fuchsinophilic (MT stain) (d) Immunohistochemistry for Myoglobin showed positivity in these casts MT: Masson’s Trichrome.
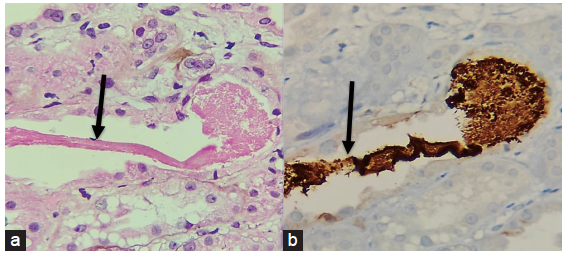
- A panel of photomicrographs (400x) of the renal biopsy with black arrows showing the (a) ropy and granular cast which is eosinophilic (H/E stain) (b) Immunohistochemistry for Myoglobin showed positivity in the cast.
Rosuvastatin and dapagliflozin were discontinued. In view of AIN, a tapering dose of steroid was prescribed. Within a few weeks, liver functions and renal functions normalized and he became dialysis independent. Rosuvastatin was rechallenged after two months and his liver functions, renal functions, and CPK levels have remained normal.
Discussion
The American Heart Association (AHA) recommends using SGLT2i and statins to reduce heart failure hospitalizations and mortality in type 2 diabetics with acute coronary syndrome. Despite the benefits, a few case studies have revealed that a combination of SGLT2i and statins enhances the risk of rhabdomyolysis.3
Rhabdomyolysis is a severe form of muscle injury characterized by extremely high levels of CPK, up to tenfold, and/or myoglobinuria and eventually renal failure.4
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and have been linked to myotoxicity or statin-associated muscle symptoms (SAMS). The myotoxicity is attributed to polymorphisms of genes involved in metabolism, transport or action of statins, production of anti-HMGCR antibodies, and reduction in Coenzyme Q10 (ubiquinone) essential for myocyte energy production.1
Unlike atorvastatin and simvastatin, which utilize the hepatic cytochrome-P450 pathway, pharmacokinetics of rosuvastatin is regulated by drug transporters. The organic-anion-transporting-polypeptides (OATPs) facilitate absorption and distribution of rosuvastatin, whereas P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporter enhance renal, biliary, and intestinal elimination.5
All SGLT2i are competitive substrates of P-gp and few have been linked with rhabdomyolysis.6,7 Proposed mechanisms involve interactions between SGLT2i and statins via P-gp, BCRP, and OATPs. SGLT2i-mediated transporter protein inhibition raises plasma statin levels and lowers statin elimination. SGLT2i’s intrinsic potential to cause sarcopenia may exacerbate SAMS.7
A few case reports have described the occurrence of myopathy and rhabdomyolysis in patients on statins, co-prescribed canagliflozin, or empagliflozin.2
Brailovski et al. reported the occurrence of rhabdomyolysis in a 76-year-old woman within three days of adding canagliflozin to a preexistent rosuvastatin therapy. She developed elevated creatinine phosphokinase levels (13,263 IU/L), hepatocellular injury, AKI, and a 15 fold elevation of rosuvastatin levels. A pharmacogenetic analysis identified a heterozygous polymorphism in genes coding for BCRP, an efflux transporter, which could have raised rosuvastatin levels. Clinical and laboratory abnormalities were promptly resolved after discontinuing canagliflozin and rosuvastatin.8 Kabadi et al. and Gao et al. also highlighted the temporal association between onset of myopathy and elevated CK levels within weeks of administering empagliflozin in patients already receiving atorvastatin and resolution of symptoms following discontinuation of empagliflozin.9,10
Another case report described severe rhabdomyolysis with a combination of ticagrelor, statins, and amlodipine, indicating that taking numerous CYP3A4 inhibitors may also exacerbate myotoxicity.4
Meanwhile, a pharmacovigilance study by Gravel et al. reported 456 cases of myopathy, including 77 cases of rhabdomyolysis, with concurrent use of statin and SGLT2i. Although, the analysis did not support the association between a higher risk of myotoxicity and this combination, the study had limitations of reporting and confounding bias and missing data.11
In our patient receiving ticagrelor and rosuvastatin with normal renal functions, dapagliflozin was recently added. He developed severe AKI, elevated CPK levels, myoglobinuria, and histopathological features diagnostic of rhabdomyolysis. This temporal association of occurrence of rhabdomyolysis following the addition of dapagliflozin to rosuvastatin raises concerns regarding enhanced myotoxicity with the drug. Although statins and SGLT2i have been identified as individual culprits, this is the first case in literature wherein dapagliflozin addition to preexistent rosuvastatin therapy was followed by rhabdomyolysis.
Conclusion
Though it remains speculative, the most probable explanation for rhabdomyolysis could be an increased exposure to statin metabolites, given the possible drug interaction between dapagliflozin and rosuvastatin. While rarer causes of rhabdomyolysis such as mitochondrial disorders or inherited gene mutations were not investigated, this case encourages us to revisit pharmacology and be cautious of the gamut of drug-drug interactions and their consequences.
Acknowledgments
We would like to thank our colleagues Dr. Sundar Sankaran, Dr. Ravindran T K, Dr. Babitha, and our families for their never-ending support and encouragement.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Statin-related muscle toxicity: An evidence-based review. touchREV Endocrinology. 2022;18:89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Myopathy associated with statins and SGLT2 – A review of literature. Curr Probl Cardiol. 2020;46:100765.
- [CrossRef] [PubMed] [Google Scholar]
- AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e895-e1032.
- [CrossRef] [PubMed] [Google Scholar]
- Severe rhabdomyolysis due to presumed drug interactions between atorvastatin with amlodipine and ticagrelor. Case reports in Crit Care. 2017;2017:3801819.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Investigating transporter-mediated drug-drug interactions using a physiologically based pharmacokinetic model of rosuvastatin: Investigating DDI using a rosuvastatin PBPK Model. CPT Pharmacometrics Syst Pharmacol. 2017;6:228-38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos. 2010;38:405-14.
- [CrossRef] [PubMed] [Google Scholar]
- Potential drug-drug interaction between sodium-glucose co-transporter 2 inhibitors and statins: Pharmacological and clinical evidence. ADME-Tox. 2021;17:697-705.
- [CrossRef] [PubMed] [Google Scholar]
- Rosuvastatin myotoxicity after starting canagliflozin treatment: A case report. Ann Intern Med. 2020;173:585-7.
- [CrossRef] [PubMed] [Google Scholar]
- Marked weight loss, muscle wasting and fatigue on administration of empagliflozin in a subject with type 2 diabetes. BJMMR. 2017;21:1-7.
- [CrossRef] [Google Scholar]
- Myopathy secondary to empagliflozin therapy in type 2 diabetes. Endocrinol Diabetes Metab Case Rep. 2020;2020:20-0017.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Concomitant use of statins and sodium glucose co-transporter 2 inhibitors and the risk of myotoxicity reporting: A disproportionality analysis. Br J Clin Pharmacol. 2023;89:2430-45.
- [CrossRef] [PubMed] [Google Scholar]








