Translate this page into:
Skin Microcirculatory Changes in Relation to Arteriovenous Fistula Maturation
Address for correspondence: Dr. Siew Cheng Chai, Reconstructive Sciences Unit, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, Kubang Kerian, 16150 Kelantan, Malaysia. E-mail: scchai513@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Maturation of arteriovenous fistula (AVF) involves complex vascular remodeling. In this study, we evaluated the changes of skin microvascular perfusion over the extremity with AVF maturation using the laser Doppler fluximetry (LDF). A total of 45 patients with chronic kidney disease, Stages IV–V, were included; they had undergone AVF creation from July 2014 to June 2016 at our institute. The measurement of skin microvascular perfusion was accomplished proximal and distal to the fistula anastomosis site: pre- and post-operative day 1, week 2, week 6, and week 12. Thirty-two patients with mean age of 55.6 had achieved AVF maturation. There were 40.6% radial-based and 59.4% brachial-based AVF. There was a 32.8% reduction of mean skin perfusion distal to the fistula by day 1 compared to the baseline perfusion; however, perfusion increased 47% by week 2 compared to day 1 and no dramatic change was subsequently noted. There was an increase of mean skin perfusion, proximal to fistula anastomosis, over 12 weeks with 35.8% at day 1 from the baseline. However, the changes of the mean skin perfusion were not statistically significant. There was no significant relation of skin perfusion changes with the type of fistula, diabetes mellitus, hypertension, and hyperlipidemia. LDF successfully detected the subclinical change of skin microvascular perfusion in relation to AVF creation. Reduction of skin perfusion distal to the fistula suggests that in patients with existing perfusion inadequacy of extremities, they may experience ischemic symptoms as early as day 1 postoperation, and require close monitoring for distal limb ischemic-related complications.
Keywords
Arteriovenous fistula
microvascular perfusion
renal failure
steal phenomenon
Introduction
Chronic kidney disease (CKD) is a global public health burden. According to the 2010 Global Burden of Disease study, CKD was ranked 27th in the list of causes of a total number of deaths caused worldwide in 1990; however, this rank reached to 18th in 2010.[1] The number receiving renal replacement therapy is estimated to be more than 1.4 million worldwide, with incidents annually growing by approximately 8%.[2] The introduction of hemodialysis (HD) in 1943 for patients with advanced kidney failure had improved the life expectancy.[3]
Upper extremities native arteriovenous fistula (AVF) is preferred vascular access for maintaining HD compared to the arteriovenous graft and central venous catheter due to lower complication rates and health-care expenditures.[4567] Matured AVF is needed to support the HD blood circuit. The National Kidney Foundation Disease Outcome Quality Initiative guidelines suggest that an “adequate” AVF should fulfill some anatomical and functional parameters which include vein diameter >0.6 cm, flow over venous limb >600 ml/min, and located approximately 0.6 cm from the skin surface.[4] Allon and Robbin[8] showed that the mean primary failure of AVF ranged from 2% to 53% based on conducted studies between 1977 and 2002.
Maturation of AVF involves the diversion of blood flow from the feeding artery to the low resistance vein, which subsequently leads to complex vascular remodeling. Mean blood flow in an artery must increase to at least 500 ml/min to obtain successful AVF.[9] Blood flow for matured radial- and branchial-based AVFs can be 500–2000 ml/min and 500–3000 ml/min, respectively.[10] Several researches were done to understand the mechanism of fistula maturation and its relation to access-related complications. The changes of skin microvascular perfusion concerning AVF maturation and its relation to the occurrence of access-related ischemia are still undetermined. Therefore, in this study, we used the laser Doppler fluximetry (LDF) to capture the skin microcirculation changes that occur distal and proximal to the anastomotic site of AVF throughout its process of maturation; this is because LDF may be used as an adjunct tool or monitoring tool for postoperative care of AVF.
Subjects and Methods
This prospective study was conducted in Hospital Universiti Sains Malaysia (HUSM), Kubang Kerian Kelantan to evaluate the changes of upper extremities’ skin microvascular perfusion with AVF creation and maturation in patients with CKD Stages IV–V. Ethical approval for this study was obtained from the Human Ethical Committee of Universiti Sains Malaysia (USM/JEPeM/140394). All participants provided their written informed consent, and the study was performed according to the principles stated in the Declaration of Helsinki.
All patients with CKD Stages IV–V underwent preoperative vascular assessment, including clinical Allen test and ultrasound vascular mapping, to determine the vessel's suitability for AVF creation. A total of 45 patients with CKD Stages IV–V who were electively scheduled and admitted in the surgical ward under the Reconstructive Sciences Unit, HUSM for AVF creation from July 2014 to June 2016 were included in this study based on the inclusion and exclusion criteria, as follows
Inclusion criteria
-
Patients with Stages IV–V CKD (glomerular filtration rate <29 ml/min)
-
Adult patients, 18 years old and above
-
Consented for AVF creation
-
Compliant to follow-up.
Exclusion criteria
-
Symptomatic peripheral vascular disease or ABSI <0.4
-
Preexisting central vein stenosis
-
Vasculitic disease
-
Dermatological disease, which involves the upper extremities, for example, dermatitis and psoriasis.
To achieve standardized measurement, all procedures were carried out in the morning at Pharmacology Vascular Laboratory, HUSM. The protocol of the study was standardized for every subject. Measurements were conducted in a quiet room with controlled temperatures of 24°C ± 1°C. Patients refrained from drinking coffee and eating a high salt diet for 12 h before measurements. Patients should not perform the heavy exercise for 1 day and rested for 10 min before each measurement. Post surgery, patients were given sufficient pain control.
All consented patients had one visit preoperation (day 0) and another four visits postoperation (day 1, week 2, week 6 and week 12) to the Pharmacology Vascular Laboratory. A single operator conducted all procedures in the morning from 8 am to 12 pm. During the first visit, measurements of anthropometry (height and weight), blood pressure, pulse, and skin microvascular perfusion were carried out. For subsequent follow-ups, measurements of the blood pressure, pulse, and skin microvascular perfusion were accomplished. Maturation of AVF was assessed by postoperation during week 6 by using Duplex Ultrasound. Maturation is defined as the created fistula that fulfils the parameters, which include a vein diameter >0.6 cm, flow over venous limb >600 ml/min, and located approximately 0.6 cm from the skin's surface. Only patients with matured AVF on a 6-week follow-up were subjected to a follow-up at week 12. Patients with failed or delayed matured AVF were excluded from the study, as these patients would require secondary procedures.
For the measurement of skin perfusion, a dual channel LDF – DRT4 (Moor Instrument, Axminster, U.K) with two DP1T-V2 laser skin probes were used. A molded, flexible white probe holder (PH1-H2) with its pad consisting of double-sided adhesive discs was employed to stabilize the placement of each probe to the skin during measurements.
Measurements were recorded with patients in the supine position. Bilateral upper limbs were at the side of the body and stabilized with hand supporters. Two skin probes (VP1T/7) were employed for taking measurements at the operated and nonoperated upper limbs. At the operated upper limb, 1 probe was placed 3 cm distal to the wrist creases (at the thenar region of the palmar surface of the hand), and 1 probe was placed 2 cm away from the anastomosis site. For the nonoperated upper limb, the site of placement for the probe was the same as the operated limb [Figure 1]. The placement area for the probe was cleaned with an alcohol impregnated swab. Measurement of each limb was carried out for 5 min. The mean of the skin microcirculation over 5 min was presented in unit AU. Data were analyzed with IBM SPSS V22.0 (International Business Machine Corp., Armonk, New York, United State) using repeated measures ANOVA statistics.

- Placement of the skin probe over the limb during measurement
Results
Demographic details
Of 45, 32 patients had successful, matured fistulas and completed 12 weeks of post operative follow-ups. Thirteen patients were excluded from the study. Eleven patients had primary fistula failure, and two patients defaulted from the follow-ups.
In the group of patients with matured fistulas, there were 20 males and 12 females with the mean age of 55.6 ± 10.6. About 93.8% of patients had CKD Stage V, and 17 patients had begun dialysis before the creation of the fistula. Premorbidities of patients were summarized in Table 1. Mean ± standard deviation of blood pressure, pulse rate, and skin temperature of patients between each visit were not statistically significant [Table 2]. There were 13 (40.6%) radial-based and 19 (59.4%) brachial-based AVF.
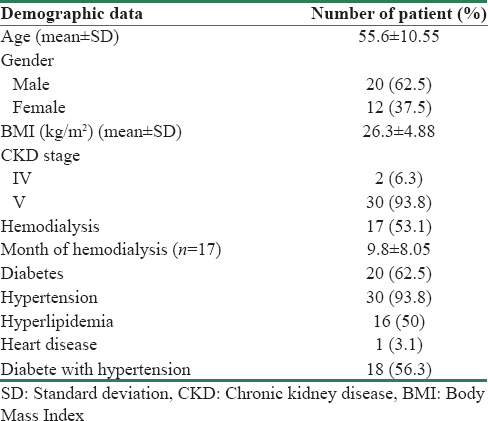

Skin microvascular perfusion over distal hand (distal to arteriovenous fistula anastomosis)
For the skin perfusion over the hand (distal to AVF anastomosis), the preoperative baseline measurements (day 0) for the operated limb and the control limb were 41.7 ± 5.4 AU and 42.0 ± 5.1 AU, respectively. On day 1 postoperation, the hand skin perfusion over the operated limb reduced by 32.8% (28.0 ± 4.9 AU). By week 2 postoperation, the hand perfusion increased to 41.3 ± 6.3 AU. When AVF achieved maturation by week 6 postoperation, there was a reduction of hand perfusion by 23.3% (32 ± 4.2 AU) compared to the baseline measurement (day 0). By week 12, hand skin perfusion was lower compared to the baseline (day 0) at 37.5 ± 5.1 AU. The changes of the skin microvascular perfusion distal to the AVF were not statistically significant with the maturation of the fistula. In the control upper limb, there was no dramatic reduction of hand skin perfusion on day 1 (41.6 ± 5.6 AU). By weeks 6 and 12, the hand perfusion of the control limb had slightly increased compared to the baseline; to 46 ± 5.4 AU and 49.2 ± 7.6 AU, respectively. There was no statistically significant change to the skin hand perfusion between the operated limb and the control limb before and after AVF creation and maturation (p = 0.485).
Skin microvascular perfusion over operation site (proximal to arteriovenous fistula anastomosis)
For the skin perfusion over the operation site (proximal to AVF anastomosis), the day 0 measurements for the operated limb and control limb were 12.3 ± 1.1 AU and 14.0 ± 1.7 AU, respectively. On day 1 postoperation, both the operated and control limbs displayed an increment of skin perfusion by 35.7% (16.7 ± 1.3 AU) and 16.4% (16.3 ± 2.8 AU). In the operated limb, the skin perfusion increased compared to the baseline for week 2 to week 12 postoperation, with range of perfusion at 17.0 ± 1.3 AU and 19.9 ± 2.7 AU. However, in the control limb, skin perfusion at the site proximal to anastomosis reduced compared to day 1, and was back to near baseline with ranges at 13.3 ± 1.5 AU and 14.8 ± 1.4 AU. There was no statistically significant change in the operated limb and the control limb in relation to fistula maturation (p = 0.195). The trend of skin perfusion changes distal and proximal to AVF anastomosis with maturation are summarized in Table 3 and Figure 2.
There was no significant change of skin perfusion with the type of fistula (p = 0.059) and diabetes mellitus (p = 0.148). None of the documented patients have access-related ischemia symptoms.

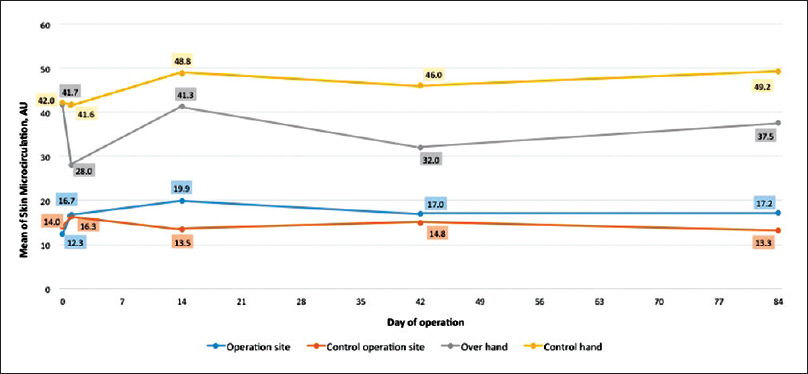
- Trend of skin microvascular perfusion changes over time
Discussion
Hemodynamic changes in AVF are one of the key components in achieving fistula maturation. The pressure gradient and total resistance of the fistula circuit (including the feeding artery, fistula anastomosis, and downstream vein) influences the fistula flow rate. Blood flow over a matured fistula may achieve 500–2000 ml/min for forearm fistula and 500–3000 ml/min for upper arm fistula to accomplish efficiency for HD.[10] According to Dixon,[9] blood flow over the artery must increase 10–20 fold for fistula maturation. Once AVF is created, the blood from the feeding artery will shunt to low resistance venous limb. We are always in doubt about the perfusion to the upper limb, especially the distal extremity, after the creation and maturation of AVF. In addition, there is no guideline concerning specific flow rate across the fistula to correlate with the reduction of perfusion to the distal extremity. Therefore, using LDF in our study, we managed to capture the trend of skin microvascular perfusion changes that are proximal and distal to anastomosis over time. These changes were not statistically significant since they were expected to be subclinical during the process of maturation of AVF. None of our patients documented symptoms of access-related ischemia. The reduction of skin perfusion at the distal extremity on day 1 was most profound (32.8% from the baseline), which can be due to the immediate shutting of blood flow across the fistula while awaiting compensation dilatation of the proximal artery to increase the blood flow. This finding was supported by the result from the control limb where there was no drop in distal circulation. By 2–12 weeks postoperation, the distal skin perfusion had returned near to the baseline. This correlated well with previous studies[1112] which showed the gradual increase of mean arterial blood flow from preoperation to 12 weeks in conjunction with AVF maturation. Increase of blood flow over the artery helps restore the perfusion over distal extremities.
Access-related hand ischemia is a potentially devastating complication for CKD patients. The incidence of clinically significant access-related distal extremity ischemia may range from 1% to 10%.[131415] Access-related ischemic is due to the interaction of a few contributing factors (i) insufficient inflow due to the failure of remodeling inflow artery/stenosis; (ii) high distal vascular resistance/atherosclerotic arterial system, with or without reverse flow distal to anastomosis; (iii) high flow fistula; and (iv) intradialytic hypotension/systemic hypotension.[1516] The predisposing risk factors for access-related ischemia include age >60, female sex, diabetes mellitus, a history of multiple AVF creation over the same limb, and branchial-based AVF.[151718] Prompt diagnosis and treatment are needed to prevent permanent ischemic damage. However, the possible timing occurrence of this complication as well as the duration for postoperation surveillance, are still underdetermined. In a retrospective study, Lazarides et al.[19] reported that patients with AV bridge grafting developed access-related ischemia immediately after the operation and required close monitoring within 24 h; whereas, ischemia following brachial-based AVF can occur between days to years. Therefore, to correlate with our findings, access-related ischemia may occur as early as day 1 postoperation for high-risk patients since there is a profound drop of distal skin perfusion. The reduction of distal skin perfusion is expected to increase near the baseline by week 2 postoperation due to vascular remodeling. Any dramatic reduction of distal skin microcirculation from the baseline and the failure for it to recover in a timely manner can be the maker of distal limb ischemia. This finding suggests that patients require closer monitoring or further investigation to promptly detect access-related ischemia before profound ischemic damage to distal upper extremity can occur.
In addition, studies indicated that 77%–100% of radial-based AVF demonstrated postoperative steal phenomenon which involves the retrograde flow towards the anastomosis at the distal artery.[202122] Brachial-based AVF was reported to have a higher rate of access-related ischemia.[17] We found that patients with functional AVF would have some degree of subclinical reduction in distal skin microcirculation that may be related to the steal phenomenon since patients’ skin perfusions were below the baseline at the 12-week follow-up. However, none of our patients developed symptoms or signs of access-related ischemia; as proposed, the steal phenomenon does not play a pathophysiological role in access-related hand ischemia.[16] There was also no significant difference in the reduction of skin perfusion over the distal extremity in patients with brachial-based or radial-based AVF.
LDF was chosen to be the tool of measurement for skin microcirculation in this study as it is a noninvasive, reproducible and reliable method to access the microcirculation of the skin. It can provide continuous or near-continuous records.[23] In 1975, Stern had demonstrated the laser Doppler technique for the assessment of skin blood flow in humans.[24] Throughout the years, LDF has been widely used in researches and clinical practices related to cutaneous blood flow. In the medical field, literatures showed that LDF is a reliable tool for diagnosing vascular related diseases, for example, Raynaud's phenomenon[25] and peripheral vascular disease.[26] In the surgical field, LDF is well-established for its use in monitoring-free flaps transfer, replants, wound healing, burn depth and post tissue expander expansion monitoring. Studies[2728] indicated that the use of LDF allows early detection of vascular complications in free flaps surgeries with continuous monitoring before clinical signs as well as increasing the flap salvage rate.
In this study, we had conducted the procedure in a quiet room with a controlled temperature to reduce the variability in the measurements and results. We also suggest that the patient's upper limb should be stabilized with the use of thermoplastic splint, as in our study. Moreover, the blood pressure and temperature of patients should be stabilized for every follow-up.
We would suggest that LDF be used as an adjunct tool in managing patients who plan for AVF creation. Preoperative patients should have a baseline measurement for skin microcirculation over distal extremities, which is important for serving as a guide for subsequent follow-ups. LDF can further be employed in postoperative surveillance to follow-up with those at high risk for developing access-related ischemia. Based on this study, we plan to conduct a study of larger scale research on skin perfusion in relation to AVF to identify the sensitivity of LDF. We will also focus on different groups of patients, including patients with preexisting peripheral vascular disease; which was excluded in this study. Finally, LDF will be proposed for use as a monitoring tool for patients with access-related ischemia that are scheduled for revascularization procedures.
Conclusion
LDF successfully detected subclinical changes of skin microcirculation in relation to the creation and maturation of AVF. However, further studies are needed to increase the clinical use of LDF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Special thanks to Universiti Sains Malaysia for funding (Grant No. 304/PPSP/61313035).
References
- Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260-72.
- [Google Scholar]
- Chronic renal diseases as a public health problem: Epidemiology, social, and economic implications. Kidney Int Suppl. 2005;98:S7-10.
- [Google Scholar]
- DOQI clinical practise guidelines for vascular access. Am J Kidney Dis. 2006;48:177-247.
- [Google Scholar]
- Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int. 2002;61:305-16.
- [Google Scholar]
- Type of vascular access and mortality in U.S. Hemodialysis patients. Kidney Int. 2001;60:1443-51.
- [Google Scholar]
- Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40:611-22.
- [Google Scholar]
- Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int. 2002;62:1109-24.
- [Google Scholar]
- Hemodialysis vascular access survival: Upper-arm native arteriovenous fistula. Am J Kidney Dis. 2002;39:92-101.
- [Google Scholar]
- Non-invasive evaluation of vessels by duplex sonography prior to construction of arteriovenous fistulas for haemodialysis. Nephrol Dial Transplant. 1998;13:125-9.
- [Google Scholar]
- Effect of haemodynamic variables on surgically created arteriovenous fistula flow. Nephrol Dial Transplant. 1997;12:1684-8.
- [Google Scholar]
- Blood flow dynamics in patients with hemodialysis access-induced hand ischemia. J Vasc Surg. 2013;58:446-510.
- [Google Scholar]
- Haemodynamic basic for the diagnosis and treatment of angioaccess-induced steal syndrome. Adv Vasc Surg. 2000;8:147-59.
- [Google Scholar]
- Haemodialysis access-induced distal ischaemia (HAIDI) is caused by loco-regional hypotension but not by steal. Eur J Vasc Endovasc Surg. 2012;43:218-23.
- [Google Scholar]
- Steal in hemodialysis patients depends on type of vascular access. Eur J Vasc Endovasc Surg. 2006;32:710-7.
- [Google Scholar]
- Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74:8-10.
- [Google Scholar]
- Indications for surgical treatment of angioaccess-induced arterial “steal“. J Am Coll Surg. 1998;187:422-6.
- [Google Scholar]
- Characterizing flow distributions in AV fistulae for haemodialysis access. Nephrol Dial Transplant. 1998;13:3108-10.
- [Google Scholar]
- Importance of bidirectional arterial flow in dialysis fistula construction. J Cardiovasc Surg (Torino). 1979;20:395-8.
- [Google Scholar]
- Incidence of the radial steal syndrome in patients with brescia fistula for hemodialysis: Its clinical significance. J Vasc Surg. 1986;4:144-7.
- [Google Scholar]
- Reproducibility of different laser doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods. 2005;52:286-92.
- [Google Scholar]
- In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254:56-8.
- [Google Scholar]
- Laser-doppler measurement of digital blood flow regulation in normals and in patients with raynaud's phenomenon. Acta Derm Venereol. 1983;63:43-7.
- [Google Scholar]
- Laser doppler flowmetry in evaluation of lower limb resting skin circulation. A study in healthy controls and atherosclerotic patients. Scand J Clin Lab Invest. 1988;48:621-6.
- [Google Scholar]
- Monitoring free flaps using the laser doppler flowmeter: Five-year experience. Plast Reconstr Surg. 2000;105:55-61.
- [Google Scholar]
- Free flap monitoring using simultaneous non-invasive laser doppler flowmetry and tissue spectrophotometry. J Craniomaxillofac Surg. 2006;34:25-33.
- [Google Scholar]







