Translate this page into:
Spectrum of acute kidney injury in critically ill patients: A single center study from South India
Address for correspondence: Dr. M. Eswarappa, Department of Nephrology, M. S. Ramaiah Medical College, MSRIT Post, MSR Nagar, Bengaluru - 560 054, Karnataka, India. E-mail: manasnephro2002@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute kidney injury (AKI) is common in intensive care unit (ICU) and carries a high mortality rate. Reliable and comparable data about the clinical spectrum of AKI is necessary for optimizing management. The study was conducted to describe epidemiology, etiology, clinical characteristics and outcome of AKI in critically ill patients without pre-existing renal disease, diagnosed using RIFLE criteria. We retrospectively analyzed data of 500 adult patients admitted to ICU with AKI or who developed AKI in ICU. Patients with pre-existing renal disease, renal transplant recipients were excluded. AKI was predominantly encountered in older males. Diabetes, hypertension, coronary artery disease were the most commonly prevalent comorbidities. Sepsis was the most common cause of AKI, accounting for 38.6% of patients. 24.4% belonged to risk class, 37.0% to injury class, 35.0% to failure class, 3% to loss and 0.6% to ESRD class of the RIFLE criteria. Renal replacement therapy (RRT) was required in 37.2% (n = 186) of patients. About 60% recovered complete renal function. Chronic kidney disease (CKD) was a sequel in 2.4% (n = 12) of patients. Average duration of ICU stay was 5.6 days. Crude mortality rate was 37.6% (n = 188). In critically ill patients without pre-existing renal disease, elderly age, male sex, type 2 diabetes along with a primary diagnosis of sepsis were most commonly associated with AKI. Majority of the patients’ recovered complete renal function.
Keywords
Acute kidney injury
critically ill patients
end stage criteria
failure
India
injury
loss
risk
spectrum
Introduction
Acute kidney injury (AKI) is a new consensus term, encompassing a range of kidney diseases of acute onset.[123] Two trials, namely program to improve care in acute renal disease (PICARD) and beginning and ending supportive therapy (BEST) for the kidney, confirmed that AKI is a significant contributor toward mortality, morbidity among ICU patients.[456] RIFLE classification scheme and acute kidney injury network (AKIN) classification scheme have been proposed to achieve early diagnosis of AKI.[7] A new consensus definition merging the criteria has also emerged from the Kidney Disease Improving Global Outcomes.[8] With the application of these criteria, prevalence of AKI in ICU setting is >40% if sepsis is present and the mortality rates varied from 15% to 60%.[910] Longer hospital stay and economic burden are inevitable. In contrast to western literature, few reliable statistics are available regarding AKI in India.[11] We retrospectively evaluated patients with AKI, using the RIFLE criteria, to answer questions regarding most susceptible population, etiology, role of dialysis, outcomes and relation of mortality rate with RIFLE class.
Subjects and Methods
The study was conducted by Nephrology Department of our teaching hospital, a tertiary level referral center in South India, during the period of May 2011 to October 2012. A total of 500 patients with AKI during this period, who met our study requirements were evaluated retrospectively. A total of 8621 patients were admitted to the ICU during this period.
Definitions
AKI was defined as patients whose serum creatinine and/or urine output fulfilled the RIFLE criteria. Risk class was defined as increase in serum creatinine ≥1.5 × baseline or urine output <0.5 ml/kg/h for the duration ≥6 h. Injury class was defined as increase in serum creatinine ≥2.0 × baseline or urine output <0.5 ml/kg/h for ≥12h. Failure class was defined as increase in serum creatinine ≥3.0 × baseline or an absolute serum creatinine 4 mg/dl or urine output <0.3 ml/kg/h ≥24 h or anuria ≥12 h. Loss was defined as complete loss of kidney function >4 weeks, requiring dialysis. ESRD was defined as complete loss of kidney function, requiring dialysis for >3 months. Oliguria was defined as urine output below 500 ml/day. Patients who fulfilled RIFLE criteria within 48 h of admission were classified as community acquired AKI (CAAKI) and patients who fulfilled RIFLE criteria 48 h after admission were classified as hospital acquired AKI (HAAKI). Complete renal recovery was defined as estimated glomerular filtration rate (eGFR) returning to a value of ≥60 ml/min/1.73 m2 within 3 months. Chronic kidney disease (CKD) was defined as persistent reduction in eGFR after 3 months with a value <60 ml/min/1.73 m2. Mortality was defined as patients expiring during the hospital stay.
Inclusion and exclusion criteria
All patients aged above 18 years admitted to ICU with AKI and those who developed AKI after admission to ICU, during the period of May 2011 to October 2012, were included in our study. Patients with preexisting renal disease and those who received renal transplantation were excluded from the study. A total of 500 patients met the above requirements and were evaluated retrospectively.
Data collection
We retrospectively retrieved the required data from the patient files in medical records department using a standardized proforma developed for the study. Demographic information retrieved included age, sex, weight, height, duration of ICU and hospital stay. Clinical data retrieved included primary diagnosis, past medical history, presence of co-morbidities, surgical status, physical examination findings, lab investigations, treatment history, hospital course and need for renal replacement therapy (RRT). Baseline and peak levels of serum creatinine, urine output were collected. Data regarding laboratory investigations were collected to confirm the etiology of AKI, which included complete urine analysis, metabolic panel, lipid profile, blood culture, hematologic profile, coagulation profile. Radiological tests, serological tests and renal biopsy findings when available were also retrieved. Follow-up data regarding their renal function was also collected. We then analyzed the pooled data to come to our conclusions. The duration of follow-up was 3 months. There was no loss to follow-up of the patients. Statistical calculations were done using Chi-square test as and when required. P < 0.05 was considered to be significant. The calculations were carried out using SPSS (statistical package for the social sciences) 16.0 statistical software (IBM SPSS, Chicago, Illinois) when required. We compared our results to existing data available using PUBMED database.
Results
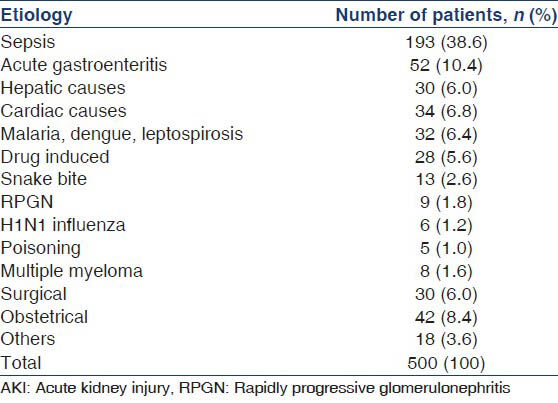
Etiology
Among the patients (n = 500) enrolled in our study, most common cause of AKI was sepsis. Urinary tract infections were the most common source of sepsis, followed by respiratory infections and diabetic foot. Gastroenteritis was the second most common cause of AKI in our study. Obstetrical diseases were the third most common cause of AKI while surgical, hepatic and cardiac disease constituted the other major causes. Malaria, dengue, leptospirosis, Drug induced AKI, snake bite, H1N1 influenza, Multiple myeloma, RPGN were the other causes of AKI, although less common. Hair dye poisoning accounted for 3 cases, organophosphates accounted for two cases. The observations regarding the etiology of AKI in our study are summarized in Table 1.
Epidemiology
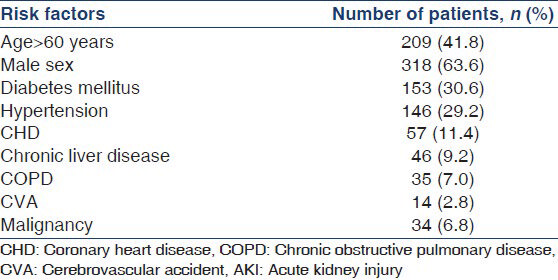
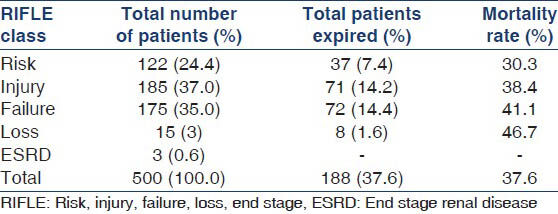
One-fourth (n = 125) of the patients developed HAAKI and the remaining presented with CAAKI. Majority of patients with AKI belonged to the age group of 61-70 years accounting for 22.8% (n = 114) of total patients [Figure 1]. The median age of the patients was 55.5 years, lying in the age group of 51-60 years. Figure 1 illustrates these observations. AKI was more commonly associated with male sex, 63.6% (n = 318) of the patients were males. Type 2 diabetes was the most common comorbidity followed by hypertension and coronary artery disease. Chronic liver disease, chronic obstructive pulmonary disease, nephrolithiasis, cerebrovascular accidents, malignancy were the other common comorbidities. About 38.6% of patients with AKI did not have any comorbidities. Table 2 summarizes these observations.
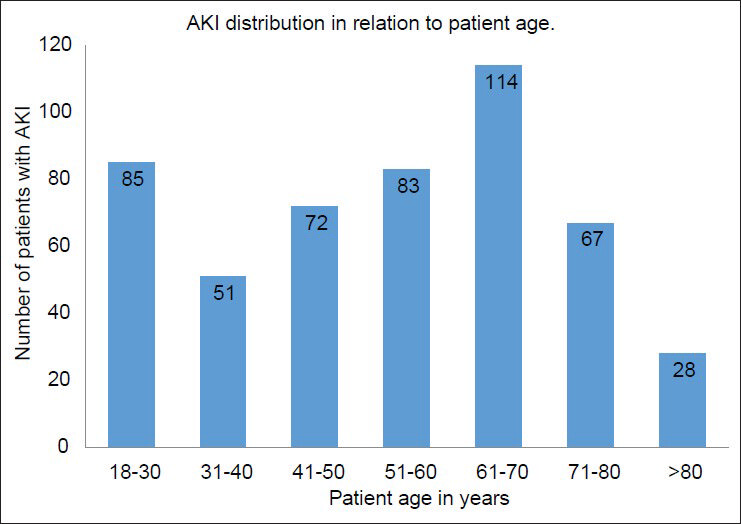
- Distribution of acute kidney injury in relation to patient age in our study
Clinical characteristics
Hypotension was the most commonly associated clinical finding, prevalent in 74.0% (n = 370) of the patients. 67% (n = 335) of the patients were oliguric and 33% of the patients non-oliguric. Non-oliguric patients had faster renal recovery. Fever, tachycardia, hypovolemia, edema were seen in 52.0% (n = 260), 37.0% (n = 185), 30.0% (n = 150) and 28.0% (n = 140) of the patients respectively. Flapping tremor was seen in 10.0% (n = 50) of the patients. The other commonly associated findings were pallor (20.0%, n = 100), icterus (21.0%, n = 105), cyanosis (5.0%, n = 25).
Outcome
Average duration of ICU stay was 5.6 days. 37.2% (n = 186) of patients in the study group required RRT during their stay in ICU. Majority of the patients who required RRT, received intermittent hemodialysis and slow low efficiency dialysis. Table 3 illustrates these observations. The median duration of dialysis requirement was 6 days. This varied with etiology. Acute gastroenteritis (GE), drug induced AKI required shorter duration of dialysis, median 3 days. Sepsis, obstetric causes, acute pancreatitis required longer duration of dialysis, median 9.5 days. 68% (n = 340) of the patients required inotropic support. Among them, 72 patients required 3 or more inotropic drugs. 61 out of these 72 patients expired. The remaining 11 developed severe ATN requiring dialysis for a prolonged period. 60% (n = 300) of the patients recovered complete renal function based. 2.4% (n = 12) of the total patients developed CKD. The crude mortality rate was 37.6% (n = 188). 91% (n = 171) of the deaths were in patients who developed AKI due to medical causes. 2.6% (n = 5) of the deaths were among surgical causes of AKI and 6.4% (n = 12) of deaths among obstetrical causes of AKI. There was a statistically significant difference between the mortalities observed with each of these diagnostic group (P = 0.02). The observed mortality in surgical and obstetrical group were less than the expected value while observed mortality in medical group was higher than expected. Table 4 illustrates the mortality outcome in relation to diagnostic class. Among patients receiving RRT, 41.5% (n = 78) patients expired in comparison to 35.03% (n = 110) deaths seen in patients not receiving RRT. There was no statistically significant difference in mortality between the two groups (P = 0.12). Figure 2 illustrates these findings. The observed mortalities in relation to RIFLE class are demonstrated in Figure 3 and Table 5. The difference in mortality rate between different RIFLE classes did not reach statistical significance (P = 0.2).

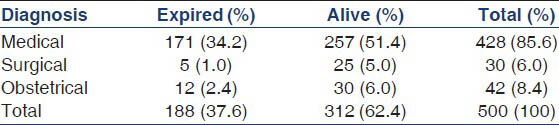
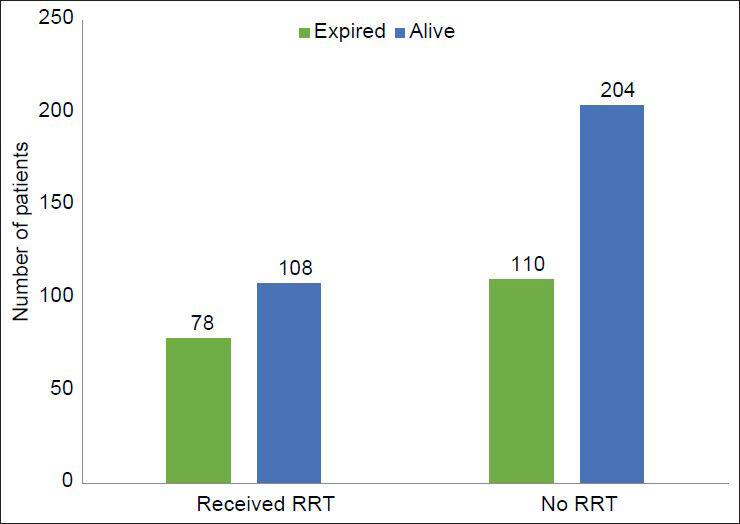
- Mortality in relation to renal replacement therapy
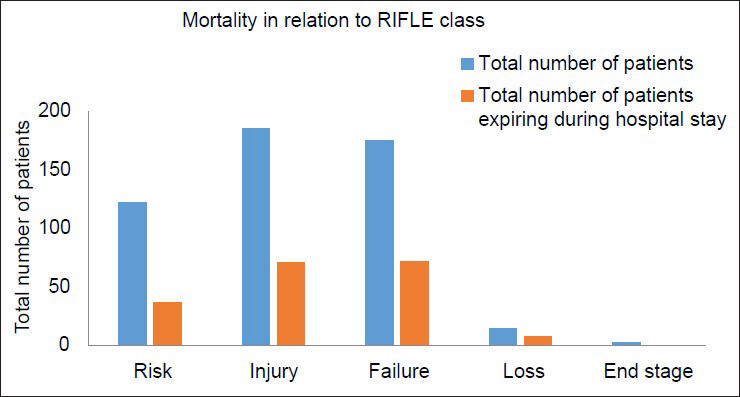
- Mortality in relation to risk, injury, failure, loss, end stage class
Discussion
AKI is a frequently observed clinical syndrome in intensive care unit (ICU), with overall incidence of 20-50%, associated with the mortality rate over 50%.[12] Paudel et al., in a prospective observational study of 100 critically ill patients in New Delhi, India showed that the incidence of AKI is 17.3 cases per 100 person years.[13] Joannidis et al. in their study involving 16,784 ICU patients, found that the prevalence of AKI in ICU was 28.5% using the AKIN criteria and 35.5% using the RIFLE criteria.[14] A study by Bagshaw et al. in Australia, involving 120,123 patients found the prevalence of AKI to be 36.1%.[9] This highlights the significant risk AKI poses to critically ill patients.
Risk factors
AKI was predominantly seen encountered in elderly age and male sex in our study. Two large studies have shown the median age of AKI in critically ill patients to be 63 and 67 years respectively.[1415] Although gender, race have not been shown to alter AKI susceptibility in previous studies, age has been consistently shown to be a risk factor.[16] A study based in United Kingdom showed 16% of the AKI patients were older than 65 years while a study in United States showed that 12.4% of the patients were above the age of 65.[1718] In accordance with previous literature, our study highlights the potential risk of AKI in critically ill, elderly patients especially above the age of 60 years. Majority of patients with AKI had preexisting comorbidities, type 2 diabetes, hypertension, coronary artery disease being the three most common. Liver, lung and neurological diseases, although less common were the other significant comorbidities seen in the patient population. These findings are in agreement with a review study by Rodrigo et al., which demonstrated a significantly increased risk of AKI in critically ill patients with older age, diabetes, hypertension, higher baseline creatinine, heart failure, sepsis/systemic inflammatory response syndrome, use of nephrotoxic drugs, higher severity of disease scores, use of vasopressors/inotropes, high risk surgery, emergency surgery.[16]
Etiology
Sepsis was the most common cause of AKI in our study accounting for more than one third of the cases. The role of sepsis in AKI has been well documented in western literature, causing nearly 50% of the AKI cases in few studies.[192021] Jha et al., and Prakash et al., which evaluated AKI irrespective of ICU setting had shown that nephrotoxic drugs were the most common cause of AKI.[2223] A recent Indian study by Singh et al., found that although nephrotoxic drugs were the most common cause of AKI in the medical ward, sepsis was the most common cause in surgical ward and ICU.[24] A study by Sandeep et al., which evaluated acute renal failure in ICU, found sepsis to be more common than nephrotoxic drugs.[25] Kaul et al., in their study have reported acute diarrhea as the most common cause.[26] In our study drug induced AKI accounted for only 5.6% of the cases, which is lower than the previous studies. This might suggest that increasing knowledge of precautions regarding nephrotoxic drugs have helped to reduce the incidence of drug induced AKI. The most common source of sepsis in our study was urogenital. This is in contrast to a multicenter, multinational study by Bagshaw et al., found that the predominant sources of sepsis were chest and abdominal (54.3%) with urogenital sepsis accounting for only 4.1% of septic AKI.[20] However, a study in India by Shah et al., found urogenital infection to be the most common source of sepsis in patients who developed AKI.[27] Acute GE was the second most common cause of AKI in our study. The higher number might be attributed to the fact that our institution is a tertiary care center, managing patients referred after developing renal complications. Malaria, dengue, leptospirosis and snake bite accounted for 9.0% (n = 45) of the cases of AKI. These represent a unique etiology spectrum of AKI in our country in comparison with the western literature.
RRT was required in 37.2% (n = 186) of the patients in our study. Previous Indian studies by Prakash et al., and Singh et al. reported that 34% (n = 28) and 20.58% (n = 7) of the cases respectively, required RRT.[2324] However, these studies did not specifically evaluate AKI patients in ICU but studied them irrespective of the hospital setting. Intermittent hemodialysis and slow low efficiency dialysis were the most commonly used modalities of RRT in our hospital. Mahajan et al., who evaluated ARF in ICU, reported that 71.1% required RRT and that intermittent RRT was the most common mode used.[25] These modes are associated with lower cost, lesser adverse effects when compared to the other RRT modes. However, patient outcomes of all three modalities have been shown to be comparable in several studies.[28] In our study, although the mortality rate was higher among patients receiving RRT, there was no statistically significant difference (P = 0.12). On the other hand, Chertow et al., previously demonstrated that among critically ill patients, acute renal failure requiring dialysis is an ominous condition with a high risk of in-hospital mortality.[29]
Outcomes
The total mortality rate in our study was 37.6% (n = 188). Several large scale studies conducted world-wide have established that AKI identified by RIFLE criteria in critically ill patients is associated with statistically significant increase in mortality.[171930] Joaniddis et al., in their study of ICU patients showed that the mortality rate among patients classified to have AKI by RIFLE criteria was 36.5%.[14] In the study by Ali et al. involving 474 patients with AKI, the in hospital mortality was found to be 32.7%.[19] However, this study involved AKI patients irrespective of the hospital setting. Two Indian studies, one using RIFLE criteria and other using AKIN criteria to diagnose AKI patients in ICU, found the mortality rate to be as high as 73.5% (n = 25) and 93.9% (n = 31) respectively.[2431] In our study, 60.0% (n = 300) of the patients recovered normal renal function. Excluding the patients who passed away, the percentage of complete renal recovery was 96.15% among the survivors. Complete renal recovery is less commonly reported in the literature, however few studies have previously shown that the majority of patients recover sufficient renal function with one study even showing 68.0% of patients recovered completely.[1921] The excellent recovery seen in our study, might be due to the exclusion of patients with preexisting renal disease. Schiffl, in his study showed that if critically ill patients with normal renal function prior to the renal insults, survive the precipitating cause of ATN, the overwhelming majority will recover sufficient renal function.[31]
A study by Bagshaw et al.,[30] which included 120,123 critically ill patients, found that 36.1% of the patients had AKI according to RIFLE criteria, with 16.2% in the risk class, 13.6% in the injury class and 6.3% in the failure class. They also reported that the mortality rates were 17.9% in the risk group, 27.7% in injury group and 33.2% in failure group. Interestingly in our study, the higher number of patient belonged to injury class, followed by failure and risk classes. This might be due to the fact that our hospital is a tertiary referral center where most of the referred patients presented in more severe stages of renal injury. The observed mortality rates in our study were highest in the failure class, followed by injury and risk classes, but the difference did not reach statistical significance. This might be attributed to the fact that primary diagnosis and underlying etiology of renal injury are equally important predictors of mortality in addition to RIFLE class. Mortality has been shown to be associated with the number of organ failure.[25] However, our study did not include critical illness scoring systems to further stratify patients, which limits our observation.
Our study has both strengths and weakness. A limitation of our study is that it is a retrospective analysis of the data. However, we evaluated 500 critically ill AKI patients, which is a significantly higher number compared to the previous Indian studies, increasing the validity of our observations. As our study involved patients who were admitted to ICU with AKI and who developed AKI in ICU, our data encompasses CAAKI as well as HAAKI. By excluding patients with prior renal disease, we eliminated the potential confounding this adds to the study, ensuring that our study population, represents those who developed AKI for the first time.
Conclusion
Sepsis was the most common cause of AKI in the critically ill patients of our study. Age >60, male gender were prevalent in the majority of AKI patients. More than 60% of the patients had associated comorbidities, with type 2 diabetes, hypertension and coronary artery disease being the three most common. Majority of the patients did not require RRT and were treated conservatively. About, 60% of the total patients recovered normal renal function, with 2.4% of the total patients developing CKD. Crude mortality rate among patients with AKI in our study group was 37.6%.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Acute kidney injury in the intensive care unit. Bosn J Basic Med Sci (10 Suppl 1):S8-12.
- [Google Scholar]
- Acute renal failure-Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-12.
- [Google Scholar]
- The epidemiology of severe acute kidney injury: From BEST to PICARD, in acute kidney injury: New concepts. Nephron Clin Pract. 2008;109:c188-91.
- [Google Scholar]
- An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and management of acute renal failure in the ICU patient: An international consensus conference in intensive care medicine. Am J Respir Crit Care Med. 2010;181:1128-55.
- [Google Scholar]
- Acute kidney injury and renal replacement therapy in the intensive care unit. Nurs Crit Care. 2009;14:207-12.
- [Google Scholar]
- Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- [Google Scholar]
- Definition and classification of acute kidney injury. Kidney Int. 2012;2(1 Suppl 2):19-36.
- [Google Scholar]
- Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care. 2008;12:R47.
- [Google Scholar]
- Definition and classification of acute kidney injury. Nephron Clin Pract. 2008;109:c182-7.
- [Google Scholar]
- Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013 2013:479730.
- [Google Scholar]
- Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692-702.
- [Google Scholar]
- Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813-8.
- [Google Scholar]
- Risk factors for development of acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Crit Care Res Pract 2012 2012:691013.
- [Google Scholar]
- Acute kidney injury: Epidemiology and diagnostic criteria. Curr Opin Crit Care. 2006;12:531-7.
- [Google Scholar]
- OECD (2010), “OECD Factbook 2006”, OECD Factbook Statistics (database) doi:10.1787/data-00374-en
- [Google Scholar]
- Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292-8.
- [Google Scholar]
- Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431-9.
- [Google Scholar]
- AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819-25.
- [Google Scholar]
- Spectrum of hospital-acquired acute renal failure in the developing countries-Chandigarh study. Q J Med. 1992;83:497-505.
- [Google Scholar]
- Hospital-acquired acute kidney injury in medical, surgical, and intensive care unit: A comparative study. Indian J Nephrol. 2013;23:24-9.
- [Google Scholar]
- Spectrum of acute renal failure and factors predicting its outcome in an intensive care unit in India. Ren Fail. 2006;28:119-24.
- [Google Scholar]
- Spectrum of community-acquired acute kidney injury in India: A retrospective study. Saudi J Kidney Dis Transpl. 2012;23:619-28.
- [Google Scholar]
- Renal involvement in sepsis: A prospective single-center study of 136 cases. Saudi J Kidney Dis Transpl. 2013;24:620-9.
- [Google Scholar]
- Renal replacement therapy in acute kidney injury. Adv Chronic Kidney Dis. 2013;20:76-84.
- [Google Scholar]
- Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505-11.
- [Google Scholar]
- A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569-74.
- [Google Scholar]
- Renal recovery from acute tubular necrosis requiring renal replacement therapy: A prospective study in critically ill patients. Nephrol Dial Transplant. 2006;21:1248-52.
- [Google Scholar]







