Translate this page into:
Sporadic Form of Glomerulocystic Kidney Disease in a Child: A Case Report
Corresponding author: Dr. Lesa Dawman, Department of Pediatrics, Room No. 5120, 5A, Advanced Pediatrics Centre, PGIMER, Chandigarh - 160012, India. E-mail: lesadawman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaur N, Pradeep D, Nada R, Bhatia A, Dawman L, Tiewsoh K. Sporadic Form of Glomerulocystic Kidney Disease in a Child: A Case Report. Indian J Nephrol. 2024;34:392-5. doi: 10.4103/ijn.ijn_43_23
Abstract
Glomerulocystic kidney disease (GCKD) is a rare form of cystic renal disease. We report a four-week-old baby girl born to non-consanguineous parents; their antenatal third-trimester ultrasound showed severe oligohydramnios that required amnioinfusion. Post-natal ultrasound examination showed few tiny cysts (2-3mm) involving the cortices in bilateral kidneys. Kidney biopsy showed dilatation of Bowman’s space and cystically dilated glomeruli, suggestive of GCKD. Whole exome sequencing revealed no pathogenic or likely pathogenic variant.
Keywords
Children
Glomerulocystic kidney disease
Renal failure
Ultrasound
Introduction
Glomerulocystic kidney disease (GCKD) is a heterogeneous group of disorders characterised by tiny cortical cysts; due to the dilatation of Bowman’s space.1 GCKD occurs as sporadic and familial forms. It has been seen in infants and young children who present with renal failure.2
Case Report
A four-week-old baby girl born to non-consanguineous parents, was referred for evaluation of kidney dysfunction. The antenatal third-trimester ultrasound showed severe oligohydramnios that required amnioinfusion. The baby was born at 35 weeks by emergency lower segment caesarean section (LSCS) due to cord entanglement and with a birth weight of 2.2 kg. Postnatally, she experienced respiratory distress, which was treated as early-onset neonatal sepsis and with intravenous antibiotics. She had deranged renal function tests (serum creatinine 1.2 mg/dl). At three weeks of life, she was admitted for refusal of feeds and lethargy, with a serum creatinine level of 2.8 mg/dl, which increased to 4.1 mg/dl. At admission, investigations showed hyperkalemia (serum potassium at 5.6 mEq/L), deranged renal function tests (blood urea at 119 mg/dl, serum creatinine at 5.6 mg/dl), calcium at 8.7 mg/dl, phosphorus at 6.1 mg/dl, alkaline phosphatase at 224 IU/L, serum vitamin D3 at 29 ng/ml, and serum parathyroid hormone at 218 pg/ml. Ultrasonography showed a right kidney that measured 5 cm, a left kidney that measured 5.4 cm, and a few tiny cysts (2–3 mm) that involved the cortices were seen scattered at places bilaterally and had increased cortical echogenicity. Kidney biopsy was performed and showed dilatation of Bowman’s space and cystically dilated glomeruli, both of which were suggestive of glomerulocystic disease [Figure 1]. No obvious extrarenal malformations or dysmorphic features were detected. There was no family history of cystic renal disorders. Whole exome sequencing revealed no pathogenic or likely pathogenic variant. Ultrasonography screening of parents was normal. At discharge, the serum creatinine level was 2.9 mg/dl [Table 1].
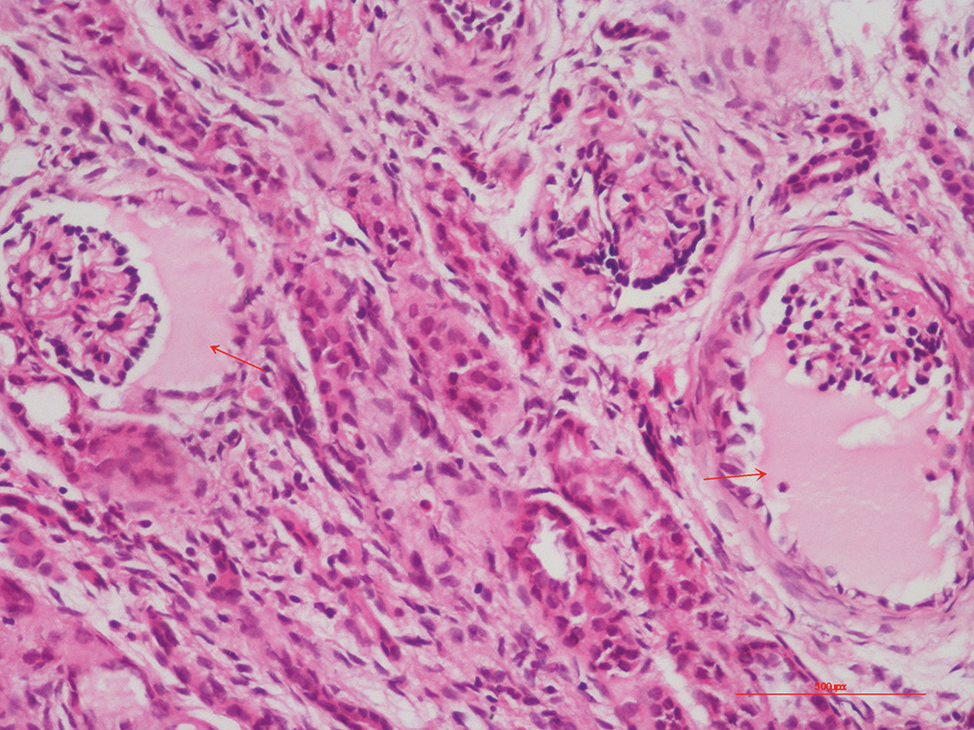
- Renal biopsy depicting markedly dilated Bowman’s space (glomerular cysts) in two glomeruli (200×, Hematoxylin and Eosin).
| Blood parameters | At birth | At admission (4 weeks) | At discharge | At 2 months | At 6 months | At 11 months |
|---|---|---|---|---|---|---|
| Blood urea (mg/dl) | 57 | 119 | 48 | 54 | 84 | 67 |
| S. Creatinine (mg/dl) | 1.2 | 5.64 | 2.9 | 2.76 | 2.09 | 1.16 |
| S. Sodium (mmol/L) | 136 | 135 | 138 | 136 | 137 | |
| S. Potassium (mmol/L) | 5.6 | 5.4 | 4.7 | 5.1 | 4.5 | |
| S. Calcium (mg/dl) | 8.7 | 10 | 10 | 10.9 | 10 | |
| S. Phosphorus (mg/dl) | 6.1 | 3.7 | 3.7 | 3.5 | 3.8 | |
| ALP (IU/L) | 224 | 496 | 496 | 210 | 210 |
S.=Serum; ALP: Alkaline phosphatase
Discussion
Cystic glomeruli in an infant was first reported by Roos in 1941.3 Taxy and Filmer1 first used the term glomerulocystic kidney disease in 1976 to describe cystic dilatation of Bowman’s space. Later, Bernstein defined glomerular cysts as the dilatation of Bowman’s space to 2–3 times of the normal size in at least 5% of glomeruli.4 Glomerulocystic kidneys can be broadly classified under three major categories: (1) GCKD, which includes sporadic forms and heritable non-syndromic forms like autosomal dominant polycystic kidney disease (ADPKD) in infants and familial hypoplastic GCKD; (2) glomerulocystic kidneys associated with heritable malformation syndromes such as tuberous sclerosis (TS), orofaciodigital syndrome type I, trisomy 13, and Zellweger cerebrohepatorenal syndrome; and (3) glomerular cystic changes in dysplastic kidneys.4 Isolated GCKD can occur as a sporadic condition, a familial disorder, or as the infantile manifestation of ADPKD. Later on, a descriptive classification of GCKD was made.2
Glomerular cysts in GCKD are usually bilateral and tiny, with average size of 2–3 mm.5 Thus, affected kidneys are of normal size or variably enlarged. Small-kidneys are seen in familial hypoplastic GCKD.6 Most cases of GCKD are seen in the neonatal period.7 It has a diverse presentation: renal failure is the usual presentation in newborns and infants, and hypertension, hematuria, or abdominal pain may be presented by older children and adolescents or may be detected incidentally.8 The different clinical presentations and the course of the disease can be explained by the fact that glomerular cysts affect only a few glomeruli. The superimposed diseases hasten the onset of renal failure or bring the patient to clinical attention.
Ultrasonography can show tiny cortical cysts, increased cortical echogenicity, and loss of corticomedullary differentiation.9,10 Contrast-enhanced magnetic resonance imaging (CEMRI) can be used to distinguish GCKD from other cystic renal diseases. Small renal cortical cysts can be seen on MRI and appear hypointense and hyperintense on T1- and T2-weighted images, respectively.11 However, we did not perform MRI. Kidney biopsy is required to establish the diagnosis of GCKD and to differentiate it from other cystic renal disorders.8 In 10% of the cases of GCKD (familial or sporadic), associated abnormalities of intrahepatic duct have been observed.4
The close differentials for GCKD are ADPKD, autosomal recessive polycystic kidney disease (ARPKD), and renal dysplasia [Figure 2]. The algorithm for screening children with kidney cysts have been described in literature [Figure 2].10 The cortical distribution of cysts differentiates GCKD from other cystic renal disorders.11 The presence of medullary cysts should raise the possibility of ARPKD, whereas tubular cysts should raise suspicion of ADPKD. In 50% of cases, GCKD in infants can be early onset ADPKD. Because there is an overlap in the clinical and pathological features of ADPKD and GCKD, the presence of renal cysts in one of the family members of a GCKD patient changes the diagnosis to ADPKD.7 In renal dysplasia, apart from cysts, abnormal development of the renal cortex and medulla is seen.
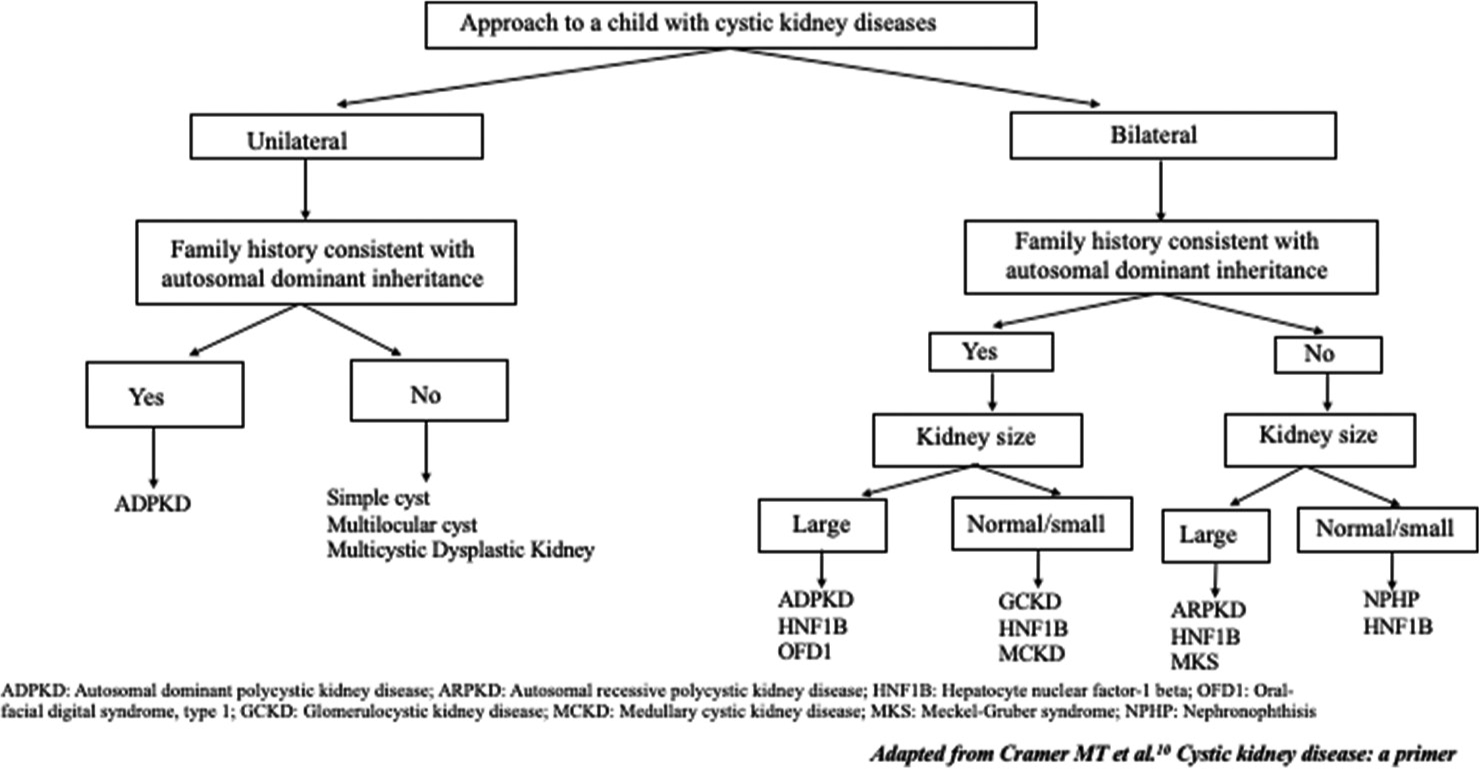
- The flow diagram for differential diagnosis of cystic kidney disease in a child.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Cystic diseases and developmental kidney defects. In: Heptinstall’s Pathology of the Kidney. Philapdephia: Lippincott Williams and Wilkins; 2007. p. :1271-2.
- [Google Scholar]
- Polycystic kidney: report of a case studied by reconstruction. Am J Dis Child. 1941;61:116-27.
- [CrossRef] [Google Scholar]
- Glomerulocystic kidney disease--nosological considerations. Pediatr Nephrol. 1993;7:464-70.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic spectrum of glomerulocystic kidneys: Report of two cases and a brief review of literature. Pediatr Pathol. 1984;2:171-86.
- [CrossRef] [PubMed] [Google Scholar]
- Familial hypoplastic glomerulocystic kidney disease: A definite entity with dominant inheritance. Am J Med Genet. 1989;34:569-73.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of glomerulocystic kidneys: a case report and review of the literature. Pediatr Pathol Lab Med. 1996;16:941-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cystic kidney diseases: many ways to form a cyst. Pediatr Nephrol. 2013;28:33-49.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of the kidneys In: National Kidney Foundation Primer on Kidney Diseases. E-Book. Philadelphia: Elsevier; 2022. p. :52-68.
- [Google Scholar]
- Cystic kidney disease: a primer. Adv Chronic Kidney Dis. 2015;22:297-305.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerulocystic kidney disease: MRI findings. Abdom Imaging. 2003;28:889-92.
- [CrossRef] [PubMed] [Google Scholar]







