Translate this page into:
Spot Urine Albumin Creatinine Ratio can be a Predictor of Cognitive Function in Type 2 Diabetes Mellitus
Address for correspondence: Dr. Deepak Kumar Panigrahi, A21, Shree Balaji Apartments, Plot 37, Sector 6, Dwarka, New Delhi - 110075, India. E-mail: drdeepaklhmc@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
In diabetes mellitus (DM), the underlying pathophysiology of albuminuria and cognitive dysfunction is similar. So, we hypothesized that urinary albumin excretion (UAE) could be linked to cognitive dysfunction in type 2 diabetes mellitus.
Methods and Materials:
It was a hospital-based observational study. Patient aged 40–60 years with type 2 DM were included in this study. Complete assessment with detailed history, physical examination, and necessary biochemical investigations including spot urine albumin creatinine ratio (uACR) was done. Cognitive status was determined in all the individuals with the application of Hindi translated version of the mini-mental status examination (MMSE) questionnaire.
Results:
In 80 patients, the mean MMSE score was 25.37 ± 3.34. Cognitive dysfunction (score <26) was present in 45% of individuals. Spot uACR, estimated glomerular filtration rate (eGFR), glycated hemoglobin (HbA1c), presence of retinopathy and dyslipidemia were significantly different between the normal and subnormal scoring groups. On multivariate analysis spot uACR was found to be independently predicting odds of developing cognitive dysfunction (OR 1.01, CI 1.004–1.022; P = 0.001). The mean MMSE scores in normo-albuminuric (n = 15), moderately increased albuminuric (n = 48) and severely albuminuric (n = 17) patients were 28.00 ± 1.60, 25.54 ± 3.33 and 22.58 ± 2.31, respectively, which were significantly different among the three groups (P < 0.001).
Conclusions:
Spot uACR could be helpful in predicting cognitive decline in people with type 2 DM.
Keywords
Albumin creatinine ratio
cognitive function
diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM), a chronic metabolic disorder is one of the most prevalent non-communicable diseases. Its prevalence has been increasing steadily all over the world.[12] If not appropriately managed, it can lead to increased morbidity and mortality because of the risks of cardiovascular, renal, and neurologic complications.[34] A number of past studies have shown that renal involvement in T2DM is early as evidenced by moderately increased albuminuria (microalbuminuria previously) before changes in serum creatinine.[56] Hence, urinary albumin excretion (UAE) has been used as a marker of early diabetes and subsequent risk stratification as far as the complications are concerned.[6] A less addressed and not well recognized complication of DM is cognitive impairment.[789] In older individuals, diabetes is a risk factor for dementia and for cognitive decline. Most studies have defined cognitive decline by a change in neuro-cognitive test scores but the clinical relevance of this information has been unclear. In addition, there have been few longitudinal studies till date stressing upon the factors influencing cognitive impairment in diabetes. The northern part of India has a very high prevalence rate of T2DM and the primary language here is Hindi. There is paucity of studies citing any link between any urine albumin creatinine ratio (uACR) and mini-mental status examination (MMSE) score especially if used in a Hindi modified version. Therefore, the aim of the present study was to find any association of uACR with cognitive function score assessment using MMSE (Hindi version).
Methods
It was a cross-sectional study. It was approved by the medical ethics committee of the institute. All the study participants were recruited only after obtaining informed and written consent after explaining them the details of the project. We recruited Hindi literate people, aged between 40-60 years with T2DM for the study. The study was carried out in accordance with the declaration of Helsinki. Exclusion criteria were type 1 or secondary DM, history of any neuro-psychiatric illness, illiterate patient, presence of any significant visual, hearing or any impairment in communication, subjects with history or examination suggestive of other risk factors known to cause cognitive impairment like hypoglycemia, chronic liver disease, serum creatinine more than 1.5 mg/dl or advanced renal disease, hypothyroidism and stroke, etc.
A complete history including name, age, sex, registration number, occupation, chief complaints, presenting and past illness, personal and family history, duration of diabetes, anti-diabetic medications, any history suggestive of micro and macro-vascular complications, smoking, alcoholism was taken in each case. A thorough physical examination including pulse, blood pressure, respiratory rate, temperature, body mass index (BMI), waist-to-hip ratio (WHR) was done. A detailed neurological examination including fundoscopy was done to look for any retinal or neurological involvement. No brain imaging was done in the included participants. All these patients were then evaluated by the MMSE scale in Hindi for the determination of cognitive score as defined below. The English version of MMSE[10] has been translated into many regional languages worldwide and successfully applied. It has also been converted to Hindi format[11] and used with similar results. In this study, MMSE was done in Hindi language. The language conversion was done by experts in the language. A pretesting was carried out in 23 individuals among the recruited who were literate in both Hindi and English language. It was observed that there was no significant difference between the results obtained in both the languages.
The scoring system in MMSE is still a matter of debate. In several studies, the researchers[12] have taken a score of ≥26 as normal MMSE scoring (as adjusted to the educational level) without any cognitive impairment while others considered ≥24 as adequate performance.[10] In this study the results were interpreted considering 26 as a cut off. Complete biochemical investigations comprising of hemoglobin (Hb), total leukocyte count (TLC), peripheral smear, liver function test (LFT), kidney function test (KFT), serum electrolytes, fasting lipids, glycated hemoglobin (HbA1c), and spot uACR were done. Spot uACR was done in the first early morning urine sample in each patient.
Data were recorded in a pre-designed proforma. SPSS statistical software was used for analysis. Comparison of various categorical variables was done by using the Chi-square test. Student's t test was used for the comparisons between group of variables with normal distributions. Unadjusted odds ratio (95% CI) was computed for each of the potential factors with the outcome. Univariate and multivariate binary logistic regression analysis was used to identify independent factors associated with cognitive function impairment. Receiver-operating characteristic (ROC) curve was plotted for the detection of uACR values that would carry significance in predicting cognitive impairment.
Results
A total of 80 patients were included in the study. The mean age of the population was 51.71 ± 7.15 years. Thirty-one (38.8%) out of 80 were males. The median duration of diagnosis of T2DM was 8.5 (IQR 5.0-11.7) years. The mean MMSE score was 25.37 ± 3.34. The median uACR value was 185.13 (IQR 88.0-289.11) mg/gm. The frequencies of other demographic variables are shown in Table 1.
| Parameters (n=80) | Frequency |
|---|---|
| Age of recipients (years) (mean±SD) | 51.71±7.15 |
| Males (n, %) | 31 (38.8%) |
| Weight (Kgs) (mean±SD) | 66.0±6.34 |
| Height (cm) (mean±SD) | 154.6±6.19 |
| BMI (Kg/m2) (mean±SD) | 27.6±2.5 |
| Waist circumference (cm) (mean±SD) | 93.4±5.3 |
| Hip Circumference (cm) (mean±SD) | 94.9±5.1 |
| Waist-Hip Ratio (mean±SD) | 0.98±0.03 |
| Duration of Diabetes (years) (median, IQR) | 8.5 (5.0-11.7) |
| Smokers (n,%) | 24 (30%) |
| Hypertension (n, %) | 28 (35.0%) |
| Diabetic Retinopathy (n, %) | 48 (60%) |
| Diabetic Neuropathy (n, %) | 15 (18.8%) |
| MMSE Score (mean±SD) | 25.37±3.3 |
| Fasting Blood Sugar (mg/dl) (mean±SD) | 147.1±23.2 |
| Serum Creatinine (mg/dl) (mean±SD) | 0.9±0.2 |
| eGFR (ml/min/1.73m2) | 82.7±21.2 |
| Spot UACR (mg/gm) (median, IQR) | 185.13 (88.0-289.11) |
| HbA1c (mean±SD) | 7.2±0.6 |
| Dyslipidemia (n, %) | 54 (67.5%) |
BMI-body mass index, MMSE-mini-mental status examination, eGFR-estimated glomerular filtration rate, IQR-Inter quartile range, UACR-urine albumin creatinine ratio, HbA1c-glycated hemoglobin
Correlation between MMSE score and laboratory variables including uACR
On further analysis, it was observed that age had a statistically significant inverse correlation (-0.267; P = 0.017) with MMSE scores. Similarly, HbA1c (-0.337; P = 0.002), Spot UACR (-0.572; P < 0.001) were also seen to be negatively correlating with MMSE scores significantly. However, the eGFR could be positively correlated to MMSE score (0.447, P = 0.001). The variables such as duration of disease, smoking history, height, weight, BMI did not have any significant correlations (P > 0.05). [Table 2]
| Variables | Correlation Coefficient | Significance level |
|---|---|---|
| Age | -0.267 | 0.01 |
| Duration of disease | -0.061 | 0.58 |
| Height | -0.041 | 0.71 |
| Weight | -0.031 | 0.78 |
| BMI | 0.005 | 0.96 |
| Waist Hip Ratio | 0.064 | 0.57 |
| eGFR | 0.447 | 0.001 |
| Spot uACR | -0.594 | 0.001 |
| HbA1C | -0.337 | 0.002 |
BMI-body mass index, MMSE-mini-mental status examination, eGFR-estimated glomerular filtration rate, uACR-urine albumin creatinine ratio, HbA1c-glycated hemoglobin
Comparison of variables
Considering MMSE score ≤26 as cut off, 45% (36 out of 80) of individuals had subnormal cognition. 42.5% had scores in the range 20-25 and only 2.5% of patients scored <20.
All the important variables were compared between the groups. The mean age was marginally higher in the group with MMSE score less than 26, however it did not attain statistical significance (P = 0.07). The frequency of incident diabetic retinopathy was higher in the group with MMSE score less than 26 in a statistically significant manner (P < 0.05). Similarly, a significant difference between the groups were seen as far as the variables like dyslipidemia, eGFR, Spot uACR, and HbA1c levels were concerned. No difference between the groups was seen with respect to sex of the individuals, duration of diabetes, BMI, or associated hypertension. [Table 3] On further analysis, MMSE scores were also found to be significantly different between normo-albuminuric and moderately albuminuric individuals (P = 0.016), between moderately albuminuric and severely albuminuric individuals (P < 0.002) and also between normo-albuminuric and severely albuminuric individuals (P < 0.001).
| Parameters | MMSE score ≥26 n=44 (55%) | MMSE score <26 n=36 (45%) | P |
|---|---|---|---|
| Age (years) (mean±SD) | 50.4±6.9 | 53.3±7.1 | 0.07 |
| Males (n, %) | 19 (43.1%) | 12 (33.3%) | 0.36 |
| Weight in Kg (mean±SD) | 65.95±6.87 | 66.05±5.71 | 0.94 |
| Height in cm (mean±SD) | 154.63±6.03 | 154.75±6.4 | 0.93 |
| BMI (kg/m2) (mean±SD) | 27.59±2.69 | 27.61±2.47 | 0.97 |
| Waist hip ratio (mean±SD) | 0.98±0.03 | 0.98±0.03 | 0.71 |
| Duration of Diabetes (years) (median, IQR) | 9.0 (4-10) | 8.0 (5-15) | 0.38 |
| Hypertension (n, %) | 14 (31.8%) | 14 (38.8%) | 0.61 |
| Diabetic Retinopathy (n, %) | 17 (38.6%) | 31 (86.1%) | 0.001 |
| Diabetic Neuropathy (n, %) | 6 (13.6%) | 9 (25%) | 0.25 |
| eGFR (mean±SD) | 90.73±17.06 | 73.05±21.97 | 0.001 |
| Spot UPCR in mg/gm (median, IQR) | 120.69 (23.4-185.9) | 286.2 (197.3-375.3) | 0.001 |
| HbA1c (mean±SD) | 7.12±0.49 | 7.48±0.71 | 0.009 |
| Dyslipidemia (n, %) | 22 (50%) | 32 (88.9%) | 0.001 |
BMI-body mass index, MMSE-mini-mental status examination, eGFR-estimated glomerular filtration rate, UACR-urine albumin creatinine ratio, IQR-Inter quartile range, HbA1c-glycated hemoglobin
On univariate logistic regression analysis, factors like associated diabetic retinopathy, dyslipidemia, eGFR, HbA1c values were found to be significant predictors of MMSE score less than 26. (P < 0.05). Spot uACR was also seen to be a significant predictor of MMSE score <26 (OR 1.01 CI 1.008-1.020; P = 0.001). However, on multivariate analysis, only associated retinopathy, dyslipidemia, and spot UACR were found to be a significant predictor of score less than 26 [Table 4]. The area under the ROC curve drawn for spot UACR for predicting cognitive dysfunction was also found to be significant (0.848 ± 0.043, CI 0.763-0.933; P < 0.05) [Figure 1].
| Parameters | Odds ratio | 95% CI | P |
|---|---|---|---|
| Age | 0.96 | 0.87-1.07 | 0.524 |
| Retinopathy | 6.05 | 1.22-30.02 | 0.027 |
| eGFR | 1.01 | 0.97-1.07 | 0.445 |
| Spot uPCR | 1.01 | 1.004-1.022 | 0.004 |
| Dyslipidemia | 9.7 | 1.91-49.28 | 0.006 |
| HbA1C | 1.9 | 0.58-6.64 | 0.278 |
CI- Confidence interval, eGFR-estimated glomerular filtration rate, uACR-urine albumin creatinine ratio, HbA1c-glycated hemoglobin
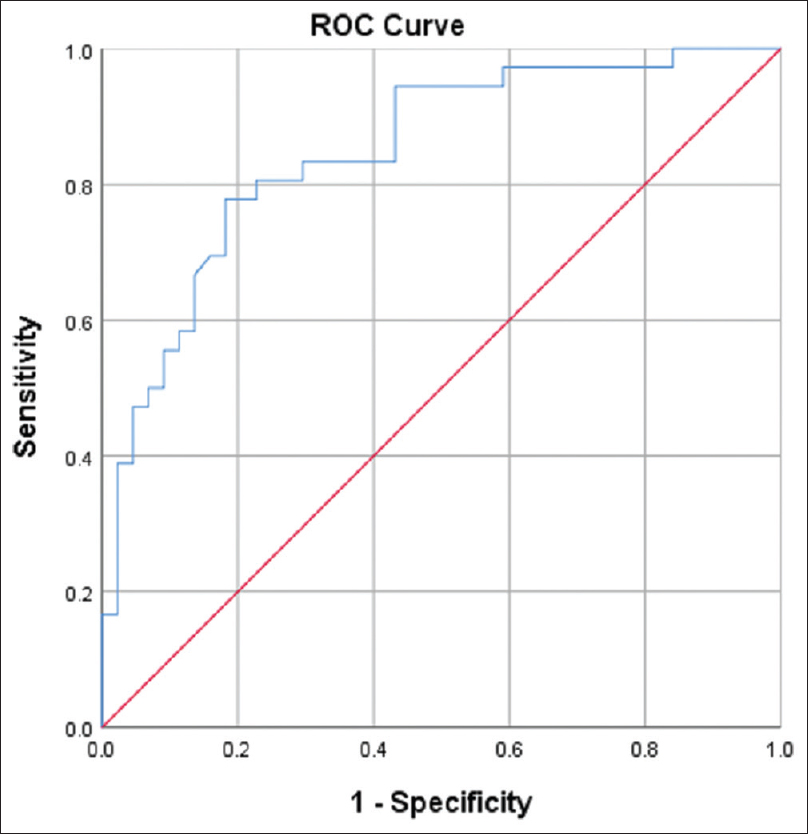
- ROC curve-Spot UACR as predictor of cognitive dysfunction
Discussion
A number of previous studies have shown that UAE does have an effect on cognitive decline in T2DM patients. Our study is crisp and comprehensive. An independent association of spot uACR with cognitive dysfunction was also observed in our study.
In our cohort, 45% of the diabetic population had cognitive impairment. Lalithambika et al.[13] found out that 54.3% of diabetic patients had mild cognitive impairment. The mean age was 53.3 ± 7.6 years. This finding was similar to that obtained in our study. In another study by Rama Mishra et al.[14] the incidence of mild cognitive impairment in the diabetic population was 44%. The age range in their cohort was 45–65 years. They also had excluded the patients with chronic kidney disease (CKD). Similarly, Elaati et al.[15] found out that 56.8% of their diabetic patients had mild cognitive dysfunction and the mean age of the population was 50.0 ± 11.7 years. In our study the mean age of the population was 51.7 years. We have excluded the aged population in view of the fact that age related cognitive decline may affect our study result as a confounder. The higher incidence of cognitive impairment in this study can be due to the lower sample size. Secondly the population studied despite literate belonged to a lower socioeconomic status. Most of the females were housewives and males were daily wagers or doing small business. So, on MMSE (a subjective score) could vary upon their sharpness. Majority (42.5%) with MMSE <26 were found to be having mild cognitive impairment only (MMSE score between 20-25).
In a multicentre retrospective study Li etal.[16] studied the correlation between cognitive impairment and renal microangiopathy in patients with T2DM patients. They demonstrated that the total MMSE score was negatively correlated with the UAE and positively correlated with the eGFR. This finding was similar to our study. However, in our study on multivariate analysis eGFR failed to predict cognitive dysfunction but spot uACR did (eGFR, OR 1.01, 95% CI 0.97–1.07; P = 0.445). This variability can be attributed to the fact that our population was relatively younger and the diabetic control status of our cohort was better as reflected by the mean HbA1c value of 7.2 ± 0.6. Also, the median duration of the diseasewas 8.5 years. So, if we follow up these patients for a longer period, they may develop a significant decline in eGFR (if having uncontrolled disease) which might then predict the cognitive status. A similar result was obtained in the study by Barzilay et al.[17] Participants were included from the Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes study (ACCORD-MIND). They found that in middle-aged adults with diabetes with preserved baseline eGFR (around 90 mL/min per 1.73 m2), cognitive function declined in participants with persistent and progressive albuminuria compared with participants without albuminuria. Bruce et al.[18] in their study on prediction of cognitive dysfunction in older diabetics found out that microalbuminuria was a risk factor for cognitive decline, whereas drugs the renin-angiotensin system inhibiting drugs were protective in preventing cognitive decline as they tend to decrease the albumin excretion. This emphasizes the mechanism linking diabetic nephropathy with diabetic cerebrovascular diseases. Their population comprised of persons older than 60 years. Similarly, a Turkish study evaluated the relationship between microalbuminuria and cognitive functions in elderly T2DM patients. However, they could not establish the link between microalbuminuria and cognitive dysfunction.[19]
In a prospective analysis of the Rancho Bernardo cohort, it was seen that albuminuria is linked to cognitive decline. A greater decline in MMSE and category fluency scores were seen in subjects with an uACR of ≥30 mg/gm.[20] These results also pointed towards a common underlying mechanism affecting the renal and cerebral microvasculature. Similar is the finding in our study. On multivariate analysis, spot uACR was significantly predicting cognitive decline, however eGFR was not.
Analysis of results from the U.S. National Health and Nutrition Examination Survey 1999–2002 showed that the presence of a higher level of UAE was inversely associated with cognitive function in participants with the peripheral arterial disease (PAD).[21] They had used 2-min Digit Symbol Substitution Test (DSST) for cognitive testing. Cognitive assessment has been done by a variety of methods including magnetic resonance imaging (MRI) to assess brain volume and to look for any evidence of small vessel disease.[2223] However, MMSE was used in our study because of the suitability. It can be done in lesser time and in outpatient basis too.
In the present study, the UAE assessed as a continuous variable was more strongly associated with cognitive decline. Although various studies have observed a close link between MMSE score and uACR, a systemic evaluation of the relationship between them is generally not carried out in routine clinical scenarios. Besides, the mechanism linking microalbuminuria with cognitive function is unclear at present. Moderate albuminuria has been associated with ischemia involving cerebral microcirculation; hence a vascular mechanism may explain its effect on cognitive decline. Additional plausible mechanisms include endothelial dysfunction and chronic low-grade inflammation.[2425] If one of the key mechanisms of brain microvascular disease is leakage of serum proteins into the brain extracellular space, in a fashion parallel to albuminuria that occurs in nephrosclerosis, then the extravasation of proteins both in kidneys and in the brain could explain the relationship between uACR and cognitive function. Both are associated with albuminuria in T2DM and may have a role in the pathogenesis of cognitive dysfunction.
Thus, it can be concluded that albuminuria might be a useful screening test for the generalized microvascular disease and, if detected, might reasonably prompt more intensive therapeutic efforts to look into evidence of endothelial dysfunction in other sites such as eyes, brain, and else?where. So, it can be viewed as a surrogate marker for generalized endothelial or microvascular involvement of the d?isease. Our study showed an association between UAE and cognitive dysfunction. Since albuminuria is an early predictor of renal involvement in diabetes (usually preceding derangement of eGFR) and an early marker of endothelial dysfunction too, it would be helpful insignaling the early stage of a process resulting in vascular disease and cognitive decline.[182021]
We also found that the presence of retinopathy and dyslipidemia significantly predicted cognitive dysfunction. Low-grade inflammation does occur in diabetics and it is positively correlated with dyslipidemia.[2627] Vascular dysfunctions in elderly patients result in reduced blood flow to the nerves and reduced endoneurial oxygen pressure. So, retinopathy, neuropathy can be associated with cognitive dysfunction. HbA1c was significantly different between normal and subnormal MMSE groups implying deranged glycemic status a fore binger of cognitive dysfunction. However, on multivariate analysis the significance was lost. Similarly, declining eGFR was associated with a decline in cognitive function. However, after controlling other variables including eGFR, uACR still could predict cognitive dysfunction significantly. In our population, mean eGFR was 82.7 ± 21.2 mL/min/1.73 m2 ruling out the confounding of advanced renal issues as a modifier of cognitive function.
Our study had several limitations. It was a cross-sectional study conducted in urban tertiary care referral center. Hence the causality and the generalized applicability of the sample in the community setting could not be ascertained. Various risk factors were assessed based upon history only and could not be supported by appropriate investigations like imaging studies for assessment of micro or macrovascular changes in brain parenchyma. Several other undocumented risk factors like oxidative stress, genetic background, diet, exercise etc., which may be playing a role in the development of cognitive impairment but remain unanswered in the presen?t study. Ours was a clinical study correlating UAE with the neurocognitive parameter. Complex investigations like measurement of oxidative stress, endothelial biomarkers were not part of our aim too. Studies with clinco-laboratory correlation could have thrown more light on the high incidence of cognitive impairment.
Our study also has some strengths. Firstly, to the best of our knowledge, our study is unique since it is the first study regarding the cognitive function and related parameters in Indian T2DM patients. The strength of the study also lies in the successful use of Hindi version of popular western cognitive scales for local use.
In conclusion, uACR is related tocognitive function in Indian type 2 diabetic patients. Clinical trials are necessary to determine whether interventions targeting albuminuria can prevent cognitive decline in type 2 diabetic patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-53.
- [Google Scholar]
- IDF Diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311-21.
- [Google Scholar]
- Complications du diabète de type 2 [Type 2 diabetes complications] Presse Med. 2013;42:839-48.
- [Google Scholar]
- Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77-82.
- [Google Scholar]
- Microalbuminuria. Implications for micro- and macrovascular disease. Diabetes Care. 1992;15:1181-91.
- [Google Scholar]
- Microalbuminuria as a risk predictor in diabetes: The continuing saga. Diabetes Care. 2014;37:867-75.
- [Google Scholar]
- Diabetes mellitus and the nervous system. J Neurol Neurosurg Psychiatry. 1998;65:620-32.
- [Google Scholar]
- Cognitive impairment in diabetic patients: Can diabetic control prevent cognitive decline. J Diabetes Invest. 2012;3:413-23.
- [Google Scholar]
- Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29:1794-9.
- [Google Scholar]
- “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98.
- [Google Scholar]
- A Hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in India. IntJGeriatr Psychiatry. 1995;10:367-77.
- [Google Scholar]
- The mini-mental state examination score and the clinical diagnosis of dementia. J Clin Epidemiol. 1994;47:1061-7.
- [Google Scholar]
- Cognitive impairment and its association with glycemic control in type 2 diabetes mellitus patients. Indian J Endocr Metab. 2019;23:353-6.
- [Google Scholar]
- Association of cognitive impairment and type 2 diabetes mellitus: A case-control study. Int JContemp Med Res. 2019;6:L32-6.
- [Google Scholar]
- Assessment of comorbid mild cognitive impairment and depression in patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13:1759-64.
- [Google Scholar]
- Positive correlation between cognitive impairment and renal microangiopathy in patients with type 2 diabetic nephropathy: A multicenter retrospective study. J Int Med Res. 2018;46:5040-51.
- [Google Scholar]
- Albuminuria and cognitive decline in people with diabetes and normal renal function. CJASN. 2013;8:1907-14.
- [Google Scholar]
- Predictors of cognitive decline in older individuals with diabetes. Diabetes Care. 2008;31:2103-7.
- [Google Scholar]
- Relationship between glycemic control, microalbuminuria and cognitive functions in elderly type 2 diabetic patients. Ren Fail. 2014;36:1258-62.
- [Google Scholar]
- A prospective study of albuminuria and cognitive function in older adults: The Rancho Bernardo study. Am J Epidemiol. 2010;171:277-86.
- [Google Scholar]
- Microalbuminuria is a negative correlate for cognitive function in older adults with peripheral arterial disease: Results from the U.S. National Health and Nutrition Examination Survey 1999-2002. J Intern Med. 2007;262:562-70.
- [Google Scholar]
- Associations of early kidney disease with brain magnetic resonance imaging and cognitive function in African Americans with Type 2 Diabetes Mellitus. Am J Kidney Dis. 2017;70:627-37.
- [Google Scholar]
- Microvascular dysfunction is associated with worse cognitive performance: he Maastricht study. Hypertension. 2020;75:237-45.
- [Google Scholar]
- Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016;17:82.
- [Google Scholar]
- Endothelial dysfunction in diabetic nephropathy: State of the art and potential significance for non-diabetic renal disease. Nephrol Dial Transplant. 2004;19:778-81.
- [Google Scholar]
- Levels of inflammatory markers and their correlation with dyslipidaemia in diabetics. J Coll Physicians Surg Pak. 2009;19:207-10.
- [Google Scholar]
- A study on cognitive decline with respect to metabolic syndrome and inflammation in elderly Indians. Neurol India. 2015;63:537-41.
- [Google Scholar]







