Translate this page into:
Successful Renal Transplantation Across HLA Barrier: Report from India
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Organ donors are sometimes found “unsuitable” due to the presence of donor-specific anti-HLA antibodies in the recipient. In recent years, improved desensitization protocols have successfully helped to overcome HLA incompatibility hurdle. We present three cases where optimum desensitization was achieved in patients with the donor-specific anti-HLA antibody (DSA) leading to successful renal transplantation. All patient–donor pair underwent HLA typing, complement dependent cytotoxicity crossmatch (CDC-XM), flow cytometry XM (FC-XM), and panel reactive antibody. If any of the three tests was positive, single antigen bead assay was performed to determine the specificity of the anti-HLA antibody (s). Patients with DSA were offered organ-swap or anti-HLA antibody desensitization followed by transplantation. Desensitization protocol consisted of single dose rituximab and cascade plasmapheresis (CP) along with standard triple immunosuppression. The target DSA mean fluorescence index (MFI) was <500, along with negative CDC-XM and FC-XM for both T- and B-cells. Three patients with anti-HLA DSA, who did not find a suitable match in organ swap program, consented to anti-HLA antibody desensitization, followed by transplantation. Mean pre-desensitization antibody MFI was 1740 (1422–2280). Mean number of CP required to achieve the target MFI was 2.3 (2–3). All the three patients are on regular follow-up and have normal renal function test at a mean follow-up of 8 months. This report underlines successful application of desensitization protocol leading to successful HLA-antibody incompatible renal transplants and their continued normal renal functions.
Keywords
Cascade plasmapheresis
desensitization
donor-specific anti-HLA antibody
HLA-incompatible
panel reactive antibody
renal transplant
Introduction
Renal transplantation is the preferred modality of treatment for patients suffering from end-stage renal disease (ESRD).[1] Chronicity of the disease, associated comorbidities, regular need for maintenance hemodialysis, and lack of suitable donor for transplantation adds to the financial and mental burden on the recipient and their families. Unlike Western countries where transplants are cadaveric, majority of kidney transplants performed in India are live-related donor transplants.[12] In India, the Human Organ Transplantation Act, 1949 (amended in 2013), governs solid organ transplantation and allows only first-degree relatives including sibling, parents, children, grandparents, grandchildren, and spouse to donate an organ.[3] However, sometimes these suitable donors may be deferred due to the presence of donor-specific anti-ABO blood group or anti-HLA antibodies in the recipient. These patients are then left with two options: paired kidney exchange or overcoming the antibody hurdle.[2] The absence of regional or countrywide organ swap network limits paired kidney exchange in India. An improved desensitization protocol has successfully helped to overcome the anti-ABO antibody hurdle since the 1980s.[4] Likewise, there have been reports from all over the world, more so in last one decade, on successful transplantation after desensitization in patients with anti-HLA antibodies.[5678]
Here, we present three cases of successful HLA-incompatible renal transplantation in patients with the donor-specific anti-HLA antibody (DSA) using desensitization protocol that included rituximab and cascade plasmapheresis (CP).
Subjects and Methods
Methods
Compatibility testing
As an institutional protocol, all patient(s) underwent kinship testing with the prospective donor(s) in accordance with Organ Transplantation Act, India [3] and compatibility testing before the transplant. The testing algorithm [9] included HLA typing of patient and donor, complement dependent cytotoxicity crossmatch (CDC-XM), flow cytometry XM (FC-XM), and panel reactive antibody (PRA). If any of the three tests was positive, single antigen bead (SAB) assay was performed to determine the specificity of the IgG-type anti-HLA antibody(s).
Donor-specific anti-HLA antibody
If the specificity of anti-HLA antibody detected by SAB was against one of the donors' HLA-antigen(s), then they were called DSA. Patients with DSA were offered organ-swap or anti-HLA antibody desensitization followed by transplantation. Patient had to sign informed written consent before the initiation of desensitization protocol.
HLA typing was performed using sequence-specific primer method by HLA-ABDRDQ Low Res kit (Invitrogen, Life Technologies Corporation, WI, USA). CDC-XM was performed using isolated donor T-lymphocytes and B-lymphocytes from peripheral blood.[10] FC-XM was performed using three-color FC (BD FACS Verse) and anti-human IgG (Jackson ImmuoResearch Laboratories, USA) after discriminating T- and B-cells using CD3 and CD22 (BD Biosciences, USA).[1112] PRA was performed using IgG Flow PRA kit (One Lambda, USA) using both Class I and Class II beads. The result was calculated using the cutoff set on the negative control. SAB was performed using Lifecodes® LSA Class I and Class II kits (Immucor, Inc., GA, USA). The strength of the antibody was measured as MFI; interpretation as “more the MFI,” “more the strength” of antibody.
Desensitization protocol
This consisted of single dose-rituximab (200 mg) administration, approximately two weeks prior to CP procedure(s). CP was performed on the apheresis equipment COM.TEC (Fresenius Kabi, Germany) as reported previously by authors.[13] About 1.5–2.0 plasma volumes were processed using pore size-based 2A filter-column (Evaflux, Kawasumi Laboratories, Japan). Each CP was followed by administration of intravenous immunoglobulin (IVIG; 100 mg/kg/dose). Induction therapy for the renal transplantation surgery consisted of triple drug regime consisting of tacrolimus, corticosteroids, and mycophenolate sodium. Tacrolimus level target was 8–12 ng/ml during first 3 months, 5–8 ng/ml from 3 to 6 months, and <5 ng/ml thereafter, prednisolone was tapered to 10 mg by the end of 3 months and 5 mg by the end of 6 months. Mycophenolate sodium was initiated at 720 mg twice daily initially and tapered to 360 mg twice daily by 6 months. Patient was also administered injection Methylprednisolone sodium, 500 mg as a single dose on postoperative day zero and injection anti-thymocyte globulin (ATG), 3 mg/kg body weight in two divided doses on postoperative day zero and two as a part of induction therapy.
Target titer
The target of desensitization protocol was to achieve <500 MFI of the DSA along with negative CDC-XM and FC-XM for both T- and B-cells.
Follow-up
Post-transplant follow-up of the patient required regular renal function test (RFT) at twice weekly for the 1st month, once weekly for the 2nd month, once in a fortnight for the 3rd month and thereafter monthly once for 12 months post-transplantation. Patients also underwent post-transplant graft biopsy for any sign of graft rejection.
Results
Three patients with DSA, who did not find a suitable match in the organ-swap program, consented to anti-HLA antibody desensitization, followed by transplantation. Tables 1 and 2 highlight demographic details and pre-transplant compatibility testing details of these three patients.

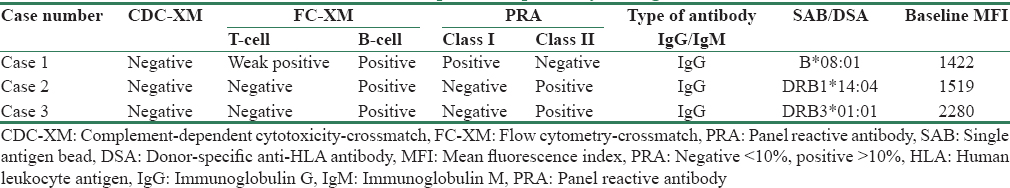
Table 3 outlines the details of CP procedures and post-desensitization compatibility testing. Post-transplant protocol graft biopsy was normal in all three cases, and no sign of rejection was reported. All three patients are on regular follow-up and had normal RFTs on each of their visits. The follow-up period for Case 1 is 10 months, Case 2 is 9 months, and Case 3 is 8 months.

Discussion
Successful HLA-incompatible renal transplants have been are reported from the US and Europe.[5678] There is no published report from India on overcoming HLA-incompatibility barrier. Authors present an initial report of three successful HLA-incompatible renal transplants with mean post-transplant follow-up of seven months.
In India, every year more than 0.17 million patients develop ESRD but only 2% undergo “desired” treatment; renal transplantation.[214] In India, the predominant transplants are from live-related donors with as few as <4% deceased donors.[15] As compared to this, the United States Renal Data System (USRDS) reports 73% as deceased donor renal transplants.[16] The Human Organ Transplantation Act, India, only allows the first-degree relative(s) to donate an organ, thus limiting the number of possible matched live-related donors. Further, many of these live-related organ donors are deferred due to either naturally occurring ABO iso-agglutinins or development of donor-specific anti-HLA antibodies in the patient. Paired Kidney Exchange (PKE) is one of the options; however, lack of knowledge, certain misconceptions, apprehension about “quality” of an organ from “other” family, concern regarding the age of donor, and lack of donor registries limit the number of such transplants. PKE transplant at authors' institute is about only 3%.[2]
ABO antibody hurdle has now been crossed and long-term graft survival and patient survival of ABO-incompatible renal transplants are now comparable to the conventional ABO-compatible renal transplant [17] world over including India. Authors' institute was one of the first centers in India to report successful ABO-incompatible renal transplant.[1819] The possibility of finding a live-related donor that could be HLA-matched but ABO-incompatible was explored but did not work out for these three patients. Therefore, HLA desensitization was planned and executed with all stakeholders; patient, donor, nephrologist, urologist, laboratory specialist, and transfusion medicine specialist agreeing to the plan. This multidisciplinary approach was one of the reasons for successful HLA incompatible transplant program.
Studies have brought out the lack of sensitivity of CDC and therefore CDC as the only test for pre-transplant workup is questioned.[20] More and more centers are adopting testing algorithm, which has two or more tests as a part of pretransplant compatibility workup. Authors' center also sees merit in such approach and therefore as a part of institutional prerenal transplant workup [9] all patient–donor pairs undergo CDC-XM, FC-XM, and PRA testing. The algorithm allows transplant straightaway if all three are negative. However, if FC-XM or PRA is positive, SAB is performed to identify the anti-HLA antibody as “donor-specific antibody” or otherwise. If it is not DSA, the transplant can be performed. However, in case of DSA, either an alternate donor has to be identified in the family or “desensitization” in the recipient, and subsequent transplant can be attempted.
Sensitization to HLA antigens due to transfusion, pregnancy, or previous transplant and subsequent development of anti-HLA antibodies is a significant barrier to successful transplantation. A strong association has been shown between DSA and hyperacute antibody-mediated rejection (AMR) and graft failure.[21] In the present report, all three cases had a history of HLA-sensitization. Case 1 and Case 3 had a history of multiple pregnancies and blood transfusion, respectively, whereas Case 2 had a history of both pregnancy and blood transfusion. However, none of the patients had a previous transplant; it was the first transplant for all the three patients.
There is unanimity in the scientific community that T-cell antibodies are detrimental to transplant success rates, and this justifies our first included patient who also had T-cell antibody in addition to B-cell antibodies. However, the opinion is divided as far as B-cell antibodies are concerned; few studies state that these have no relevance to transplant success rates [2223] while other publications have shown that the presence of cytotoxic antidonor B-cell antibodies XM in highly sensitized renal transplant patients is detrimental to graft survival.[24252627] We went by the latter school of thought and included two patients who had only B-cell antibodies.
Desensitization techniques comprising of immunosuppressants and plasmapheresis has opened new doors for these transplant-awaiting recipients by making initially “un-suitable” live-related donor, now a “suitable” donor. Better immunological understanding and improved antibody detection and identification techniques have also supported these transplants. Rituximab (anti-CD20 monoclonal antibody) was given to all the three patients, around two weeks before the initiation of CP, to inhibit the formation of new antibodies. The role of single dose rituximab as a part of induction therapy for renal transplantation has been well studied.[28] On the other hand, CP is a semiselective technique that filters-out already formed antibodies and sparing albumin and other constituents. This ensures that very little volume of plasma is wasted and therefore patients require a minimal amount of replacement. CP is an efficient and cost-effective way to decrease antibody titer leading to a successful transplant. Authors had previously successfully demonstrated the use of desensitization protocol in ABO-incompatible kidney and liver transplants.[13] The total cost to the patient of performing single CP was approximately INR 50,000 only. This included the cost of 2A filter-column (INR 30,000), PL1 kit including normal saline, 5% albumin, anticoagulant (ACD) and injection calcium gluconate (INR 18,000), and tubing set to connect the 2A filter column to PL1 kit (INR 2000). Apart from apheresis equipment (COM.TEC), no other equipment was required for the procedure. The anticipated number of procedures required would depend on the initial strength (MFI) of the antibody. The mean number of procedures done in the present study was 2.3 per patient and mean cost per patient for CP was INR 115,000.
Although the general recommendation is to keep pretransplant DSA MFI within the range of 1000–1500[29] the authors' planned target DSA as <500. This was a conscious decision to “err on the side of caution.” In their study, Lefaucheur et al.[29] concluded “as the MFI of anti-HLA DSA increased, the graft survival and the relative risk of AMR increased.” The study also reported that the prevalence of AMR increased significantly from 0.9% in patients with MFI <465 to 18.7% in those with MFI between 466 and 3000. Similarly, 1-, 3-, and 8-year graft survival correlated with anti-HLA DSA MFI: 95.0, 93.8, and 82.5% in patients with MFI <465 and 100.0, 92.1, and 78.4% for patients with MFIs between 466 and 3000.
Although the authors' report 100% graft survival and 100% patient survival at a mean follow-up period of seven months, a smaller number of cases and short-term follow-up are limitations of this study. Mayo Clinic, the USA, reported 98% 1-year graft survival and 92% 5-year graft survival in 119 HLA-incompatible renal transplant recipients [7] and National UK Registry reported 89% 3-year graft survival in 196 HLA-incompatible renal transplant recipients.[5]
Conclusion
This report underlines the successful application of desensitization protocol leading to successful HLA-antibody incompatible renal transplants and their continued normal renal functions for at least 6 months.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Outcomes of spousal versus related donor kidney transplants: A comparative study. Indian J Nephrol. 2014;24:3-8.
- [Google Scholar]
- Paired kidney exchange transplantation: Maximizing the donor pool. Indian J Nephrol. 2015;25:349-54.
- [Google Scholar]
- Legal and ethical aspects of organ donation and transplantation. Indian J Urol. 2009;25:348-55.
- [Google Scholar]
- ABO-incompatible renal transplantation: From saline flushes to antigen-specific immunoadsorption-tools to overcome the barrier. Korean J Hematol. 2011;46:164-8.
- [Google Scholar]
- UK Registry of Antibody Incompatible Transplantation 2001-2010. In: XXIII International Congress of Transplantation Society. Vancouver, August 2010.
- [Google Scholar]
- National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033-41.
- [Google Scholar]
- Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8:2684-94.
- [Google Scholar]
- Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89:1095-102.
- [Google Scholar]
- Pre-transplant anti-HLA antibody work-up of prospective renal transplant patients in a developing country: A flexible, efficient, yet cost-effective approach. Int J Immunogenet. 2015;42(5):375. doi: 10.1111/iji.12224
- [Google Scholar]
- Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-9. Bray RA, Gebel HM, Ellis TM. Flow cytometric assessment of HLA alloantibodies. Curr Protoc Cytom 2004 Feb; Chapter 6:Unit 6.16. DOI: 10.1002/0471142956.cy0616s27
- [Google Scholar]
- CLSI. Detection of HLA-specific alloantibody by Flowcytometry and Solid Phase Assay; Approved Guideline. In: CLSI document I/LA29-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- [Google Scholar]
- Cascade plasmapheresis (CP) as a preconditioning regime in ABO-incompatible live related donor liver transplants (ABOi-LDLT) Transplant Res. 2014;3:17.
- [Google Scholar]
- Five decades of Indian nephrology: A personal journey. Am J Kidney Dis. 2009;54:753-63.
- [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death 1999-2013 on CDC Wonder Online Database, Released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions Through the Vital Statistics Cooperative Program. Available from: http://www.wonder.cdc.gov/ucd-icd10.html
- [Google Scholar]
- Deceased donor organ transplantation: A single center experience. Indian J Nephrol. 2011;21:182-5.
- [Google Scholar]
- ABO-incompatible renal transplantation in developing world – Crossing the immunological (and mental) barrier. Indian J Nephrol. 2016;26:113-8.
- [Google Scholar]
- Crossmatch testing in kidney transplantation: Patterns of practice and associations with rejection and graft survival. Saudi J Kidney Dis Transpl. 2009;20:577-89.
- [Google Scholar]
- Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582-9.
- [Google Scholar]
- Clinical relevance of a positive B-cell crossmatch on renal transplantation: A multi-transplant center evaluation. Transplant Proc. 1993;25(1 Pt 1):247-50.
- [Google Scholar]
- The use of the T-cell flow cytometry crossmatch to evaluate the significance of positive B-cell serologic crossmatches in cadaveric donor renal transplantation. Transplant Proc. 1990;22:1897-8.
- [Google Scholar]
- Positive B cell crossmatches in highly sensitized patients – Influence of antibody specificity on renal transplant outcome. Transplant Proc. 1987;19(1 Pt 1):782-4.
- [Google Scholar]
- Positive B lymphocyte crossmatch and glomerular rejection in renal transplant recipients. Transplant Proc. 1987;19(1 Pt 1):785-8.
- [Google Scholar]
- Renal allograft survival in patients with positive donor-specific B lymphocyte crossmatches. Transplant Proc. 1987;19(1 Pt 1):780-1.
- [Google Scholar]
- A randomized, doubleblind, placebo-controlled, study of single-dose rituximab as induction in renal transplantation. Transplantation. 2009;87:1325-9.
- [Google Scholar]
- Living donor kidney transplantation in crossmatch-positive patients enabled by peritransplant immunoadsorption and anti-CD20 therapy. Transpl Int. 2012;25:506-17.
- [Google Scholar]
- Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398-406.
- [Google Scholar]







