Translate this page into:
Sunitinib induced nephrotic syndrome and thrombotic microangiopathy
Address for correspondence: Dr. Mahesha Vankalakunti, Pathology and Laboratory Medicine, Manipal Hospital, #98, Rustom Bagh, HAL Airport Road, Bangalore - 560 017, India. E-mail: vkmahesh123@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Sunitinib is an oral, multitargeted receptor tyrosine kinase inhibitor of targets such as vascular endothelial growth factor and platelet derived growth factor receptor. It is used for the treatment of metastatic renal cell carcinoma (RCC). Use of sunitinib has been associated with renal dysfunction and nephrotic syndrome. However, simultaneous occurrence of nephrotic syndrome and renal dysfunction in a patient treated with sunitinib is rare. We report a case of metastatic RCC treated with sunitinib for 22 months who presented with nephrotic syndrome and renal dysfunction. Renal biopsy was diagnostic of thrombotic microangiopathy with diffuse effacement of podocytic foot process.
Keywords
Nephrotic syndrome
receptor tyrosine kinase inhibitor
sunitinib
thrombotic microangiopathy
Introduction
Sunitinib is one of the standard treatment modalities for patients with metastatic renal cell carcinoma (RCC). It acts by inhibiting tyrosine kinase and affects vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) pathways. Inhibition of VEGF, either via antibody-mediated binding of the ligand or small molecule inhibition of the VEGF receptor, has demonstrated clinically relevant benefits in metastatic RCC and other solid tumors.[1–7] Use of sunitinib has been associated with hypertension, azotemia, and proteinuria in some patients.[8] We report a very rare case of thrombotic microangiopathy (TMA) leading to renal dysfunction and nephrotic syndrome developing after 22 months of sunitinib therapy in a patient who had clear cell RCC with metastatic deposits. Significant improvement in the renal function and proteinuria was noticed after withdrawal of drug and oral steroid treatment.
Case Report
A 60-year-old man was diagnosed to have right-sided clear cell RCC in June 2003. He underwent radical nephrectomy and received radiotherapy. During follow-up in November 2006, he was detected to have stomach metastases. He was managed with surgical resection and subcutaneous interferon-a for 6 months, 3 million units thrice weekly. Local disease recurrence developed in February 2008 for which oral sunitinib malate (cycle of 50 mg OD for 4 weeks followed by 2 weeks drug free interval) was started. He developed type 2 diabetes mellitus in March 2006 and was started on oral metformin. Fundoscopy did not show evidence of diabetic retinopathy; and urine was negative for proteinuria. Patient developed hypertension in April 2007 and was started on oral metoprolol 50 mg OD. Blood pressure was under control during follow-up. He developed hypothyroidism in June 2009 for which oral thyroxine 75 mg OD was introduced. In March 2010, baseline serum creatinine was 1 mg/dl.
In December 2010, he presented with history of progressively increasing edema, abdominal distension, and loss of appetite of 10-day duration. Examination revealed accelerated hypertension (BP, 180/110 mm of Hg) and anasarca. Urinalysis showed 3+ proteinuria and inactive sediments. Blood investigations revealed serum creatinine, 2.4 mg/dl; serum albumin, 2.6 g/dl; total cholesterol, 325 mg/dl; uric acid, 6.7 mg/dl; LDH, 353 U/l; and serum TSH, 42 mIU/l. Hemogram revealed Hb, 14.0 g/dl; platelet, 123 × 109/l; and total leukocyte count, 7.85 × 109/l. Peripheral smear showed normocytic normochromia and thrombocytopenia. Urine protein creatinine ratio (PCR) was 5 600 mg/g. 24-h urine protein excretion was 4 700 mg/d. USG abdomen showed normal sized left kidney that was biopsied. Biopsy revealed 19 viable glomeruli, exhibiting diffuse and global subendothelial widening with frequent microaneurysms [Figure 1]. Subendothelial spaces contained RBCs and fibrin threads. Silver stain showed double contour appearance of glomerular basement membranes without any apparent mesangial cells or endothelial cell proliferation. Tubulointerstitial compartment had insignificant findings. Swollen endothelium was noticed in the arterioles frequently. Immunofluorescence showed segmental trapping of IgM (1+) and fibrin in the capillaries in two of seven glomeruli. Remaining panel (IgG, IgA, C3, and C1q) was negative. Electron microscope examination showed markedly widened lucent subendothelial area comprising fibrin tactoids. Overlying podocytes showed diffuse effacement of foot processes [Figure 2]. Electron dense deposits were not seen.

- Glomerulus showing accumulation of plasma-like material in the widened subendothelial area globally (star mark). One of them shows presence of red blood cells in the microaneurysmal space (black arrow) at the tubular pole (×40, periodic acid-schiff methenamine silver stain)
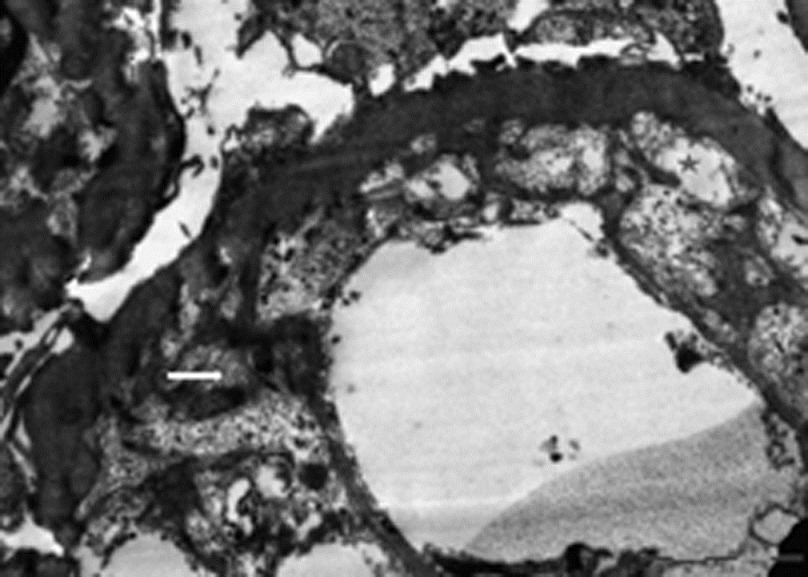
- Ultrastructure of glomerular capillary wall revealing marked widening of the subendothelial spaces by electron-lucent material (star mark) and fibrin tactoids (white arrow). New basement membrane layer is seen on the inner aspect. Overlying podocytes show diffuse effacement of foot processes (×9300, uranyl acetate and lead citrate)
Sunitinib was stopped and oral prednisolone 1 mg/kg/day was started. Thyroxine dose was increased to 125 mg OD. He continued to have nephrotic range proteinuria and renal dysfunction. Unfortunately, he presented with cough and dyspnea within 20 days of starting prednisolone. X-ray chest showed bilateral lower lobe pneumonia. Blood culture grew Streptococcus pneumoniae. Intravenous piperacillin-tazobactam was started. Renal function deteriorated and serum creatinine increased to 3.6 mg/dl. In view of severe metabolic acidosis (serum bicarbonate 12 mmol/l), hemodialysis was started. He underwent two cycles of hemodialysis after which renal functions started improving and dialysis was stopped. Serum creatinine reduced to 2.4 mg/dl. Oral prednisolone was tapered to 10 mg OD. In February 2011, his serum creatinine further improved to 2 mg/dl. Oral sorafenib (400 mg BD) was started. Prednisolone was further tapered and stopped. Latest follow-up in October 2011 showed serum creatinine, 1.5 mg/dl; serum albumin, 3.6 g/dl; Hb, 13.7 g/dl; platelet, 228 × 109/l; and urinalysis showed 1+ proteinuria with urine PCR of 600 mg/g.
Discussion
VEGF is a heterodimeric glycoprotein produced by endothelial and epithelial cells. It is also known as vascular permeability factor and enhances vascular permeability and angiogenesis associated with malignancy and wound healing.[9] It regulates endothelial cell function by induction of nitric oxide and vasodilatation and decreases vascular tone and blood pressure.[10] Boner et al. postulated that deficiency of VEGF leads to dysregulation of repair process required for normal structure, and function of glomerular capillary wall.[11] VEGF has been implicated in maintenance of glomerular filtration barrier that prevents leakage of plasma proteins into urine. Dysregulation of VEGF expression has been identified in diabetic nephropathy, thrombotic thrombocytopenic purpura, and preeclampsia.[81213]
Sunitinib inhibits multiple tyrosine kinase receptors. It affects VEGF and platelet-derived growth factor pathways. It is approved for treatment of advanced RCC. Median survival is longer in patients treated with Sunitinib than with interferon-a.[5] Inhibition of VEGF pathways is well known to be associated with proteinuria and renal dysfunction such as seen with bevacizumab, a VEGF depleting antibody.[14] Patel et al. reported seven patients with pre-eclampsia like syndrome, hypertension, and proteinuria in patients treated with sunitinib.[8] These were cured after drug withdrawal or dose modification. Muller-Deile and Schiffer described renal involvement in preeclampsia and similarities to VEGF ablation therapy.[13] Sunitinib-associated TMA was also reported by Kapiteijn et al., although renal biopsy was not done in this case.[15] First case of biopsy-proven sunitinib-induced TMA was reported by Bollee et al.[16] Eremina et al. posited that loss of VEGF from the glomerulus leads to loss of the healthy fenestrated endothelial phenotype and promotes the development of microvascular injury causing TMA.[14]
Nephrotic syndrome secondary to sunitinib is rare. Chen et al. reported first case of metastatic RCC who was treated with sunitinib and developed nephrotic syndrome and acute kidney injury secondary to acute tubular necrosis (ATN). Authors postulated that sunitinib may induce nephrotic syndrome by an immune reaction combined with amplification of underlying pathogenesis of minimal change nephropathy. This may present with transient episode of circulatory insufficiency during diuretic treatment and induce ischemic ATN.[17] Costero et al. reported a biopsy-proven TMA with focal sclerosis after 10 months of treatment with sunitinib for metastatic RCC. Patient had developed hypertension, nephrotic syndrome, and azotemia. He responded well to drug withdrawal.[18] Winn et al. reported a case of patient on treatment with sunitinib who developed renal dysfunction and acute interstitial nephritis.[19]
Treatment requires discontinuing sunitinib, which leads to recovery of renal function and reduction of proteinuria in most of the cases.[20] Hypertension has been treated variably with beta-blockers, calcium channel blockers, and ACE inhibitors. Plasma infusion has been tried successfully.[15] In the case report by Bollee et al., patient was treated with diltiazem, amiloride, and irbesartan, whereas sunitinib was continued. However, in their case, patient had mild clinical features with non-nephrotic range proteinuria and renal function was normal.[16] Chen et al. successfully treated a patient of sunitinib-induced ATN and nephrotic syndrome with drug discontinuation, temporary hemodialysis, and 4-week course of prednisolone 30 mg OD.[17] Costero et al. treated their patient with drug withdrawal, ACE inhibitor, irbesartan, and other antihypertensives.[18] Renal function normalized and proteinuria reduced to 900 mg/d from 5 400 mg/d. Our patient responded well to drug withdrawal and course of prednisolone as evidenced by reduction in proteinuria and improvement in renal function.
In conclusion, a Anti-VEGF therapy with sunitinib can induce proteinuria and nephrotic syndrome. It can also lead to TMA and renal insufficiency. In view of popularity of the treatment of RCC with sunitinib, it is advisable to periodically monitor urinary protein excretion and renal function. Those who develop nephrotic syndrome, uncontrolled hypertension, and renal dysfunction can be successfully treated with drug withdrawal, antihypertensives, and short course of oral steroids.
Acknowledgement
We sincerely thank Mrs. Tulasi Kumari, Mrs. Hema Nagaraj, and Mr. Nagaraj for their stupendous technical support in Histopathology section.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329-38.
- [Google Scholar]
- Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103-11.
- [Google Scholar]
- Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-42.
- [Google Scholar]
- Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-76.
- [Google Scholar]
- Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115-24.
- [Google Scholar]
- Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422-8.
- [Google Scholar]
- Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542-50.
- [Google Scholar]
- A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008;100:282-4.
- [Google Scholar]
- A case of membranous glomerulonephritis associated with adenocarcinoma of pancreas. Nephrol Dial Transplant. 1998;13:1049-50.
- [Google Scholar]
- Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130-5.
- [Google Scholar]
- Does vascular endothelial growth factor (VEGF) play a role in the pathogenesis of minimal change disease? Nephrol Dial Transplant. 2003;18:2293-9.
- [Google Scholar]
- Downregulation of vascular endothelial growth factor and its receptors in the kidney in rats with puromycin aminonucleoside nephrosis. Nephron. 2002;90:95-102.
- [Google Scholar]
- Renal involvement in preeclampsia: Similarities to VEGF ablation therapy. J Pregnancy. 2011;2011:176973.
- [Google Scholar]
- VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129-36.
- [Google Scholar]
- Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007;18:1745-7.
- [Google Scholar]
- Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2009;24:682-5.
- [Google Scholar]
- Nephrotic Syndrome and Acute Renal Failure Apparently Induced by Sunitinib. Case Rep Oncol. 2009;2:172-6.
- [Google Scholar]
- Inhibition of tyrosine kinases by sunitinib associated with focal segmental glomerulosclerosis lesion in addition to thrombotic microangiopathy. Nephrol Dial Transplant. 2010;25:1001-3.
- [Google Scholar]
- Biopsy-proven acute interstitial nephritis associated with the tyrosine kinase inhibitor sunitinib: A class effect? Nephrol Dial Transplant. 2009;24:673-5.
- [Google Scholar]
- Renal thrombotic microangiopathy caused by anti-VEG-Fantibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007;8:177-8.
- [Google Scholar]







