Translate this page into:
Symptomatic Bradycardia Post Rituximab Infusion
Corresponding author: Dr. Ajay Jaryal, Department of Medicine, AIIMS, Bilaspur, Himachal Pradesh, India. E-mail: drajayjaryal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jaryal A, Sidhu NS, Vikrant S. Symptomatic Bradycardia Post Rituximab Infusion. Indian J Nephrol. 2024;34:207. doi: 10.4103/ijn.ijn_167_23
Dear Editor,
A 74-year-old male with Type 2 diabetes mellitus, hypertension, subclinical hypothyroidism, and nephrotic syndrome due to biopsy-proven membranous glomerulonephritis was admitted for the second dose of rituximab. He was being treated with dapagliflozin, metformin, telmisartan, amlodipine, moxonidine, diltiazem, torsemide, metoprolol, and sodium bicarbonate. He received 500 mg of rituximab with an uneventful course up to 12 hours after the completion of infusion. His heart rate (HR) varied from 62-72/minute, and systolic blood pressure (SBP) from 150 180 mmHg. However, 12 hours later the patient complained of dizziness, and his HR and SBP were recorded to be 40/minute and 80 mmHg respectively. An ECG revealed sinus bradycardia [Figure 1]. His electrolytes and troponins levels were normal. He was managed with IV fluid and injection Atropine 1.2 mg, which increased his HR to 50/minute. All his antihypertensive medications were stopped, and after 24 hours, his HR increased to 65–75/minute, and his BP also normalized. His 2D echocardiography showed concentric left ventricular hypertrophy, a sclerotic aortic valve without significant aortic stenosis or aortic regurgitation, and normal biventricular function.
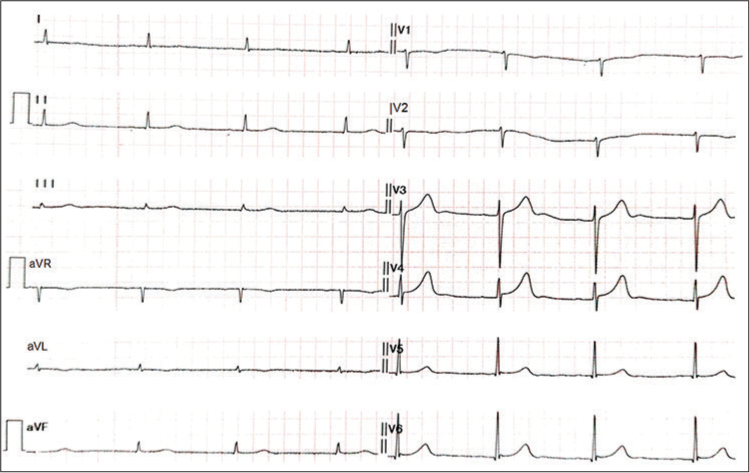
- ECG showing sinus bradycardia.
Bradycardia has rarely been reported with rituximab, both in patients with normal and abnormal conduction systems.1 It is presumed to occur due to impairment of CD20 antigen, which functions as a calcium ion channel.2 Although our patient had multiple risk factors for bradycardia such as the use of diltiazem, metoprolol, moxonidine, and subclinical hypothyroidism, yet he had a consistently normal HR, and symptomatic bradycardia only occurred after rituximab infusion. According to Naranjo adverse drug reaction probability scale, our patient has a “possible” adverse drug reaction related to rituximab infusion.3 Overall, rituximab is not significantly associated with increased cardiotoxicity.4 Our case emphasizes the need for close monitoring of cardiac arrythmias during and after rituximab infusion.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- A case of irreversible bradycardia after rituximab therapy for diffuse large B-cell lymphoma. Cardiooncology. 2020;6:22. doi: 10.1186/s40959-020-00077-5
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab-induced polymorphic ventricular tachycardia. Tex Heart Inst J. 2010;37:218-20.
- [Google Scholar]
- A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab and cardiotoxiciy. J Am Coll Cardiol. 2013;61:E582. doi: 10.1016/S0735-1097(13)60582-3
- [CrossRef] [Google Scholar]






