Translate this page into:
Systemic Lupus Erythematosus and Myasthenia Gravis: A Rare Association
Address for correspondence: Dr. J. Dhanapriya, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai - 600 003, Tamil Nadu, India. E-mail: priyamdhana@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Systemic lupus erythematosus (SLE) and myasthenia gravis (MG) are two autoimmune diseases that have a higher incidence in young females, relapsing–remitting course, and positive antinuclear antibodies. SLE and MG are two different clinical syndromes, which can coexist or precede each other; however, their occurrence in the same patient is rare. We report a 38-year-old female with biopsy-proven lupus nephritis on steroids and cyclophosphamide, later developed MG. Nerve conduction studies showed the decremental response of 15%–25% over facial muscles with no decremental response over limb muscles. Although antianticholinesterase receptor (AchR) antibodies were negative, she was treated with oral pyridostigmine 60 mg twice daily and clinical improvement of ocular symptoms was seen within 48 h. At present, she is on oral prednisolone and mycophenolate mofetil with follow-up creatinine of 1.4 mg/dl and no neurological symptoms.
Keywords
Hemodialysis
myasthenia gravis
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disorder affecting predominantly the young women characterized by multiorgan dysfunction and production of multiple autoantibodies. Myasthenia gravis (MG) is an organ-specific autoimmune disease characterized by dysfunction of the neuromuscular junction mediated by autoantibodies against the nicotinic acetylcholine receptor, the muscle-specific tyrosine kinase, or the low-density lipoprotein receptor-related protein 4, resulting in muscle weakness.[1] SLE can precede or follow the development of MG. Coexistence of SLE and MG is a rare phenomenon, and sporadic clinical cases had been reported.[2] We describe one such patient who developed MG while on treatment for lupus nephritis.
Case Report
A 38-year-old female presented on March 2016 with breathlessness, oliguria, and edema of 1-week duration. She had polyarthritis on and off for 1 year and was diagnosed to have SLE, established with 4 out of 11 criteria of the American College of Rheumatology. She had a significant obstetric history of intrauterine death at 22 weeks during her second and third pregnancy, with histopathology of placenta showing a large area of placental infarct (25%) and normal umbilical vessels. Her blood pressure was 160/100, and peripheral edema was present.
Laboratory investigation showed urinalysis: 3+ proteinuria, plenty of red blood cells; spot urine protein creatinine ratio: 8.6; blood urea: 88 mg/dl; serum creatinine: 6.4 mg/dl; serum C3:21 mg/dl; serum C4:5 mg/dl; serum antinuclear antibodies, and anti-ds DNA: positive. Anticardiolipin and antiphospholipid antibodies (APLA): negative. Ultrasonogram of the abdomen showed normal-sized kidneys. Renal biopsy showed eight glomeruli with endocapillary proliferation and lobular accentuation of the capillary tuft [Figure 1]. Early cellular crescents were identified in two glomeruli with fibrous intimal proliferation of the arteries. Immunofluorescence was positive for IgG (+3), IgM (+2), IgA (+1), C3 (+3) over capillary walls and mesangium suggestive of diffuse profilerative glomerulonephritis, lupus nephritis (ISN/RPS Class IV – A). She was treated with pulse methylprednisolone 1 g/day for 3 days, followed by oral prednisolone 60 mg/day for 8 weeks and monthly intravenous cyclophosphamide 500 mg. In addition, she also received hydroxychloroquine (HCQS) 200 mg once daily and antihypertensives. She had dialysis-dependent renal failure for 2 months and was on twice-weekly hemodialysis (HD). At 3 months after onset of renal failure, she developed difficulty in opening both the eyes. Her blood pressure was 150/100 mm Hg. Neurological examination was normal except for bilateral eye ptosis [Figure 2]. Oral HCQS was stopped, and there were no improvement even after 1 week of discontinuing this drug. The cerebral contrast-MRI and chest X-ray were normal. Serum creatinine kinase was 140 U/L. Nerve conduction studies showed the decremental response of 15%–25% over facial muscles with no decremental response over limb muscles. The findings were consistent with neuromuscular junction disorder. Anti-AchR antibody was negative. She was treated with oral pyridostigmine 60 mg twice daily. Clinical improvement was seen within 48 h. Her renal failure also slowly improved and she was dialysis independent after 4 months of HD. Mycophenolate mofetil therapy was started at a dose of 1000 mg/day orally as a maintenance therapy for lupus nephritis along with oral prednisone after 6 months. Till the last follow-up, recently, her serum creatinine was 1.41 mg/dl with no recurrence of ocular symptoms.
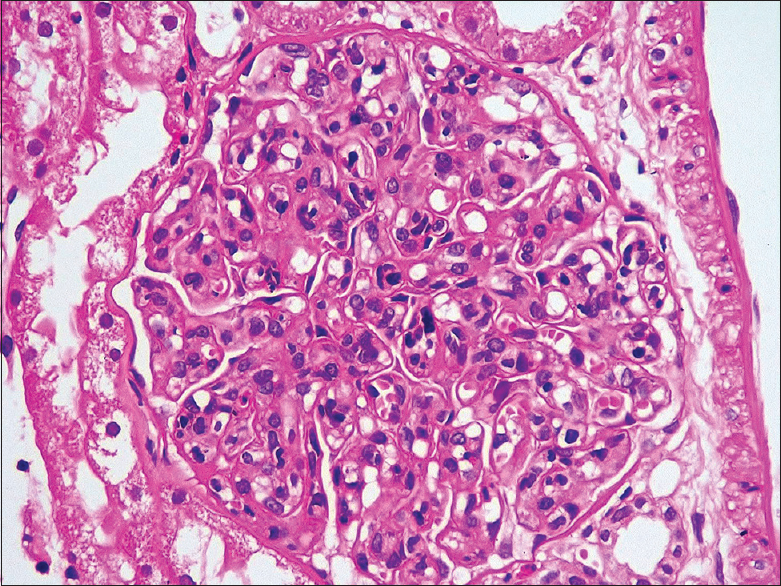
- Renal biopsy showing endocapillary proliferation and lobular accentuation of the capillary tuft (H and E)
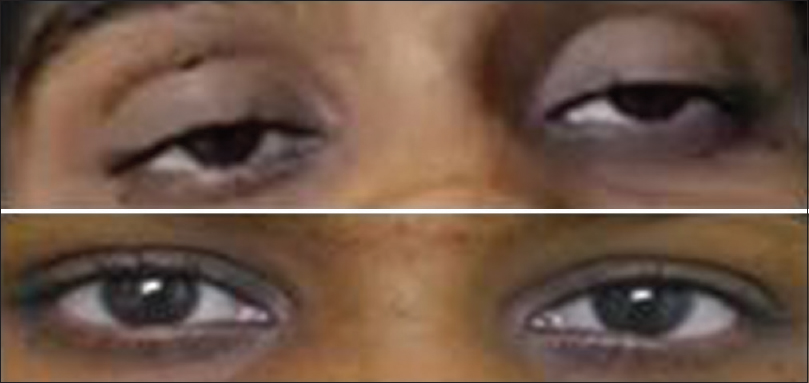
- Clinical image before and after pyridostigmine therapy showing complete improvement of ptosis
Discussion
SLE is an autoimmune disease mainly involving the skin, joints, kidneys, serosa, lungs, heart, and the nervous system. In a retrospective study of 215 patients with SLE, 30% of patients had another autoimmune disease, Sjogren's syndrome being the most common.[3] MG is a chronic autoimmune disease characterized by fatigue and proximal muscular weakness, which results from immune-mediated destruction of acetylcholine receptors at the neuromuscular junctions.[4] SLE can precede or follow the development of MG. The prevalence of SLE in MG was 2.2%–8.3% and the prevalence of MG in SLE was 1.3%.[5]
There had been 17 previously published cases of SLE and MG occurring in the same patient. SLE patients with MG seem to have less frequent cutaneous and renal manifestations.[6] In most of them, MG occurred before SLE and thymectomy was a precipitating factor for the development of SLE. The long-term thymectomized MG patients had mild T-cell lymphopenia, which was associated with hypergammaglobulinemia and B-cell hyperreactivity. In addition, many of these patients had high titers of a variety of autoantibodies, including anti-ds DNA, and anti-cardiolipin antibodies.[78]
The proposed hypothesis for association of SLE and MG is as follows: (1) loss of central tolerance after thymectomy resulting in T-cell lymphopenia with polyclonal B-cell activation, with production of antibodies, (2) molecular mimicry and structural similarity between main immunogenic region of α 65–80 of AchR and U1 small nuclear ribonucleoprotein, (3) functional defect of T regulatory cells in thymus, (4) direct effect of chloroquine over neuromuscular junction producing myasthenia like syndrome, (5) dysregulated expression of FAS (CD95) in both SLE and MG, and (6) role of chemokine CXCL13 in pathogenesis of SLE and MG. B lymphocytes play a major role in SLE, whereas T lymphocytes are critical for MG pathogenesis. CXCL13, which is a common activator of B and T lymphocytes, was responsible for the coexistence of these two autoimmune diseases.[91011121314]
The presence of ocular symptoms, persistence of ptosis even after HCQS withdrawal, and nerve conduction findings suggestive of MG prompted us to diagnose MG despite the negative AchR antibody. We started on oral anticholinesterase agents and had good clinical improvement in our patient. HCQS has a long half-life of few months and hence stopping it for 1 week may not exclude the possibility, but she had dramatic response to pyridostigmine which is more likely of MG. HCQS-induced neuromyotoxicity and myopathy usually presents with symmetrical and proximal muscle weakness and pure ocular involvement is very rare as in our patient. Although the prevalence of AchR antibodies is 80%–90%, it is only 50% in ocular myasthenia; hence, the absence of anti-AchR antibodies does not rule out MG.
Some peculiarities in our patient were that MG developed in lupus nephritis patient (only 18% of published cases had renal dysfunction), had negative anti-AchR antibodies (94% positivity in published cases), and anticardiolipin antibody and APLA were also negative (56% positive in published cases).
In conclusion, the association between SLE and MG is complex. In MG patients who had undergone thymectomy, any clinical and serological suspicion of SLE should be carefully evaluated. In lupus patients complaining of decreased muscle strength and fatiguability, the possibility of MG should be ruled out.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Anila Abraham Kurien MD, Director, RENOPATH, Chennai, for her help in the evaluation of renal biopsy.
References
- Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69:445-51.
- [Google Scholar]
- Systemic lupus erythematosus followed by myasthenia gravis: A rare association. Cent Eur J Med. 2013;8:799-802.
- [Google Scholar]
- Development of additional autoimmune diseases in a population of patients with systemic lupus erythematosus. Ann Rheum Dis. 2000;59:230-2.
- [Google Scholar]
- Association between myasthaenia gravis and systemic lupus erythematosus: Three case reports and review of the literature. Scand J Rheumatol. 2011;40:486-90.
- [Google Scholar]
- Systemic lupus erythematosus with muscle weakness due to myasthenia gravis. Acta Reumatologica Port. 2006;31:167-72.
- [Google Scholar]
- The association of systemic lupus erythematosus and myasthenia gravis: A series of 17 cases, with a special focus on hydroxychloroquine use and a review of the literature. J Neurol. 2012;259:1290-7.
- [Google Scholar]
- Appearance of systemic lupus erythematosus in patients with myasthenia gravis following thymectomy: Two case reports. J Korean Med Sci. 2004;19:134-6.
- [Google Scholar]
- High prevalence of systemic lupus erythematosus in 78 myasthenia gravis patients: A clinical and serologic study. Am J Med Sci. 2006;331:4-9.
- [Google Scholar]
- Peripheral B cell abnormalities and disease activity in systemic lupus erythematosus. Lupus. 2008;17:1064-9.
- [Google Scholar]
- Development of myasthenia gravis in systemic lupus erythematosus. EJCRIM. 2014;1 Doi: 10.12890/2014_000020
- [Google Scholar]
- Quantification and molecular characterization of regulatory T cells in connective tissue diseases. Autoimmunity. 2009;42:41-9.
- [Google Scholar]
- Antimalarial myopathy: An underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-90.
- [Google Scholar]
- Up-regulated expression of fas antigen in peripheral T cell subsets in patients with myasthenia gravis. Clin Invest Med. 2012;35:E294.
- [Google Scholar]
- The chemokine CXCL13 is a key molecule in autoimmune myasthenia gravis. Blood. 2006;108:432-40.
- [Google Scholar]







