Translate this page into:
Takayasu Aortoarteritis in Children: A Diagnostic and Management Challenge
Address for correspondence: Dr. Jyoti Sharma, Pediatric Nephrology Service, King Edward Memorial Hospital, Pune, Maharashtra, India. E-mail: jyotivsharma@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Takayasu aortoarteritis (TA) is a granulomatous inflammatory arteritis of large and medium arteries seen in young adults and rarely in children.[1] Severe hypertension because of involvement of renal arteries (RAs) is common and contributes significantly to the morbidity of pediatric TA. The diagnosis is often delayed due to its nonspecific symptoms and the disease may have a relapsing course.[2] We describe two children with TA who presented with hypertensive urgency and bilateral RA involvement. The first case presented a diagnostic challenge with hypertension and seizures, and the second had a relapsing course.
A 10-year-old girl presented with recurrent vomiting, generalized seizures, and hypertension [blood pressure (BP) 170/110 mmHg]. She had no features to suggest acute glomerulonephritis and no focal neurological deficit. Treatment was initiated with anticonvulsants and sequential addition of six antihypertensives. Her estimated glomerular filtration rate (eGFR) was 89 mL/min/1.73m2 and urine protein creatinine ratio was 13.0 mg/mg. Ultrasonography showed small right kidney (RK) with preserved echotexture and normal-sized left kidney (LK). DMSA scan revealed a single functioning LK but no reflux on micturating cystourethrogram. Renal Doppler demonstrated poor blood flow in the right RA and computed tomography angiography (CTA) showed two saccular aneurysms involving the abdominal aorta near the ostia of both RAs extending proximally to the origin of superior mesenteric artery [Figure 1a and b]. Positron emission tomography scan with 18F-flurodeoxyglucose showed hypermetabolic cervical, abdominal, and mediastinal lymph nodes, a nodular lesion in the middle lobe of the right lung, and avid lesions in the aortic wall including the aneurysmal dilatation. In view of pulmonary lesions, lymphadenopathy, and positive Mantoux test (20 mm), treatment with antitubercular drugs and oral steroids was initiated. She is on follow-up on immunosuppression for the feasibility of surgical repair after the acute inflammation is controlled.

- Case 1: CT angiogram: (a) aneurysmal dilatation of the aorta and nonopacification of the origin and proximal segment of right renal artery and (b) small right kidney when compared with left
The second case is a 15-year-old girl who presented with headache and breathlessness of 1 month's duration. Examination revealed weak pulses, BP of 100/70 mmHg in the lower limbs and 180/110 mmHg in the upper limbs, and a bruit over the right hypochondrium. Echocardiography confirmed left ventricular hypertrophy, and ultrasound (US) Doppler showed a small contracted RK with tardus flow and decreased peak systolic velocities in both RAs. The Mantoux test was 14 mm, eGFR was 54 mL/min/1.73 m2, CTA revealed narrowing of the abdominal aorta and both the main RAs, and the right RA was visualized as a thick cord. After controlling hypertension, renal angiography and left RA stenting were performed. Treatment with oral steroids and weekly methotrexate was initiated. The child became asymptomatic, normotensive on a single drug, and renal function tests became normal. She was readmitted 8 years later with similar complaints, feeble lower limb pulses, and a BP of 176/100 mmHg. Her urea and creatinine increased over 4 days to 134 and 4.0 mg/dL, respectively. On US Doppler, the RK was contracted with minimal flow in the main RA and decreased peak systolic velocity in the stented left RA. Post emergency renal angioplasty of left RA [Figure 2a and b], her renal function returned to normal and BP was controlled with a single drug. Pulse cyclophosphamide therapy was initiated and steroids were continued. Magnetic resonance angiography 4 weeks later showed focal narrowing of infrarenal abdominal aorta with good flow in left RA stent and poorly visualized right RA with collaterals.
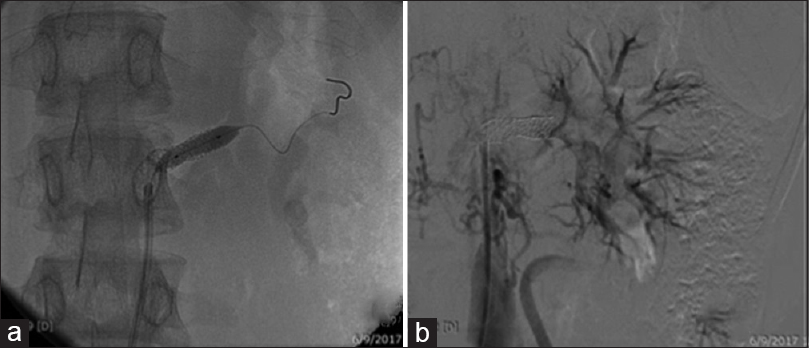
- Case 2: CT angiogram: (a) stenosed renal artery with guidewire through it and (b) revascularized kidney after dilatation
Involvement of the abdominal aorta along with RA as seen in our patients is common in Asians in TA.[3] Mantoux positivity with no other evidence of tuberculosis may be seen in children with Takayasu aortoarteritis and is postulated to be due to extensive sequence homology between mycobacterium heat shock protein (HSP) 65 and human HSP60 system. However, a higher incidence of tuberculosis in patients with TA has been reported and the need to rule out/treat tuberculosis has been emphasized in countries where TB is endemic.[4] Standard therapy of TA is immunosuppression. Biologicals like tocilizumab and infliximab have been tried in patients refractory to conventional drugs. Balloon dilatation, stenting, and/or endovascular surgery of the RA may be required in cases with critical stenosis. Restenosis rate was found to be 18.2% by surgery, 9% by angioplasty, and 62.5% with stenting.[5]
Our first patient had bilateral RA involvement with full-length right RA stenosis at presentation that was not amenable to dilatation. The second child discontinued immunosuppression and returned with stent stenosis due to ongoing inflammation that responded to urgent dilatation.
TA should be considered in the differential diagnosis when managing a child with intractable hypertension. Immunosuppressive therapy is the mainstay of management. Restenosis of dilated vessels is common because of ongoing inflammation requiring close monitoring and regular follow-up.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 2012 revised international Chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1-11.
- [Google Scholar]
- Childhood-onset Takayasu arteritis-experience from a tertiary care center in South India. J Rheumatol. 2014;41:1183-9.
- [Google Scholar]
- Clinical study of children with Takayasu arteritis: A retrospective study from a single center in China. Pediatr Rheumatol. 2017;15:29.
- [Google Scholar]
- Analysis of evidence to determine the link between Takayasu's arteritis and Tuberculosis. Indian J Rheumatol. 2015;10:2-9.
- [Google Scholar]
- Post-interventional immunosuppressive treatment and vascular restenosis in Takayasu's arteritis. Rheumatology. 2006;45:600-5.
- [Google Scholar]






