Translate this page into:
The Association Between the Levels of Serum Phosphate and Mortality Rates in Pre-Dialysis and Dialysis Patients
Corresponding author: Mehdi Noormohammad, PharmD, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. E-mail: m.nourmohammad@sbmu.ac.ir
-
Received: ,
Accepted: ,
How to cite this article: Pezeshgi A, Alemohammad Y, Tavasol A, Hajishah H, Ghasemi M, Sayadizadeh M, et al. The Association Between the Levels of Serum Phosphate and Mortality Rates in Pre-Dialysis and Dialysis Patients. Indian J Nephrol. doi: 10.25259/ijn_398_23
Abstract
Introduction:
Discovering predictors to reduce morbidity and mortality in chronic kidney disease (CKD) is now a critical global priority. Serum phosphate level is considered to be a potential marker for mortality rate in patients with CKD. Previous studies examined the independent pathogenic role of phosphorus in the development of CKD and dialysis patients but have yielded contradictory findings. This study aims at evaluating the relationship between serum phosphate levels and death rates in pre-dialysis CKD and maintenance of dialysis patients.
Method:
PubMed, Scopus, and Web of Science were searched by using MeSH term keywords. The authors did screening, data extraction, and quality assessment in accordance with the inclusion criteria. STATA 14.2 was used for statistical analyses. The analysis was performed using the random- and fixed-effects model when the heterogeneity was >50% and ≤50%, respectively. For evaluating publication bias, Funnel plots and Egger tests were used.
Result:
Eleven original studies between 2005 and 2021 met the eligibility criteria. The overall estimate of unadjusted HR of all-cause mortality each 1 mg/dL increase in the serum phosphate concentration using the random-effects model in pre-dialysis CKD and dialysis patients was 1.33 (95% CI: 0.97, 1.82, I2 = 99.1%, P = 0.074), and for adjustment, Hazard ratio was 1.27 (95% CI: 1.15, 1.39, I2 = 75.4%, P < 0.001).
Conclusion:
The findings showed the association between serum phosphate levels and death rates in pre-dialysis individuals with CKD and dialysis patients.
Keywords
Serum phosphate levels
Mortality rate
Chronic kidney disease
Dialysis patient
Meta-analysis
Introduction
Chronic kidney disease (CKD) is a rising global public health. Due to the high public health burden of CKD, the incidence of morbidity and mortality among dialysis patients is important.1,2 Some studies reported various factors playing a role in the progression of CKD and higher mortality, including nephrology care, metabolic factors like fibroblast growth factor 23 (FGF-23) and urinary oxalate, vascular stiffness, hypertension, and genetic factors.3,4
Reduced estimated glomerular filtration rate (eGFR) has been linked to an elevated likelihood of mortality, cardiovascular incidents, and the need for hospitalization in individuals with CKD.1 Many epidemiologic and interventional investigations have steadily found that rising proteinuria is a well-known prognostic marker and is linked to CKD progression, end-stage renal disease (ESRD), and death rates in both individuals with CKD and the general population.5 Proteinuria increases serum phosphate concentration through the increasing expression of the sodium-phosphate cotransporter (Na-Pi-IIa) and reduction of the biological activity of FGF-23 in the kidney’s proximal tubule, according to a recent experimental investigation.6 Additionally, individuals with low FGF-23 activity faced an increased likelihood of CKD progression.5
Vascular calcification is linked to heart-related incidents and death among individuals diagnosed with CKD stage 3–5 and people on maintenance dialysis when their serum phosphate level rises. Some previous studies have linked a reduction in serum phosphate levels to an increased risk of death due to hypophosphatemia.7
In addition, using time-dependent values, it was observed that low serum phosphate levels were significantly linked to death from any cause and mortality associated with infections, particularly in individuals over the age of 65, those on dialysis for more than a year, and those with serum albumin levels less than 3.9 g/dL.1 Finding and evaluating the relationship between mortality in CKD patients and serum phosphate levels could be an effective factor for preventing disease progression. With the increasing prevalence of patients requiring dialysis, it is critical to anticipate the risk of CKD progression to ESRD and the prevention of CKD-related mortality.8
Previous studies researching the association between serum phosphate and CKD progression and mortality had several limitations. For example, the findings did not apply to CKD patients of other ethnicities.9–11
Due to the scarcity of studies in association with predictors such as serum phosphate levels in CKD and dialysis patients as well as the contradictory findings in previous studies, the purpose of this article is to clarify and meta-analyze the exact relationship between serum phosphate levels and mortality rates in pre-dialysis CKD and maintenance of dialysis patients.
Methods
The authors utilized the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist to conduct this study.12
Search strategies
A comprehensive search was performed to find the published studies on serum phosphate levels and mortality rates in dialysis and pre-dialysis CKD patients. The following keywords and their synonyms, abbreviations, and Mesh terms were used: “Mortality,” “Morbidity,” “Serum phosphate levels,” “Dialysis,” “Pre-dialysis,” and “chronic kidney disease.” During the search process, Boolean operators “OR” and “AND” were used in a variety of combinations across international databases, including Web of Science, PubMed, Embase, and Scopus. In order to locate studies that weren’t in the aforementioned databases, Google Scholar was also searched. The references of the extracted studies were also looked at in order to find studies that might be pertinent. Duplicate records were then removed after importing all of the retrieved data into Endnote X9.
Study selection
The titles and abstracts of the remaining studies were examined to find studies that matched the following inclusion and exclusion criteria after duplicate studies had been removed: According to our inclusion criteria, the studies had to be the original cohort studies that examined the relationship between mortality rates and serum phosphate levels in patients with pre-dialysis CKD and maintenance of dialysis. These studies had to provide extractable intended data, have full texts available, and report the hazard ratio (HR) and its 95% confidence interval (CI) for mortality associated with serum phosphate levels. However, studies were disqualified if they were meta-analyses, congress abstracts, review articles, retractions, or failed to report the HR for mortality linked to serum phosphate levels. Two authors independently selected the eligible studies based on our inclusion and exclusion criteria. For any disparities, a third author assessed the studies.
Data extraction and quality assessment
Two different authors gathered information from the chosen research studies. They extracted the following data: the first author’s name, publication date, location, sample size, age of participants, CKD stage, mortality rate, body mass index (BMI), diabetes mellitus, and serum phosphate level, adjusted and unadjusted HRs of renal failure for each incremental rise in serum phosphorus levels (1 mg/dL).
A third author assessed the data for potential mistakes. Also, the Newcastle-Ottawa Scale (NOS) was employed to evaluate the methodology and quality of the conducted studies. Studies were divided, scored, and then classified into three groups of low (scores 0–3), moderate (scores 4–6), and high quality (scores 7–9), while none of the studies had a score <4.
Data synthesis and analysis
Composite HRs and their corresponding 95% CI were combined using a random-effects model. Meta-regression was employed to evaluate the relationship between serum phosphorus level and renal failure and death rates using adjusted and unadjusted log HR per unit exposure or what was estimable from category-specific estimates.
The meta-regression pooled category-specific HRs of renal failure based on divisions of serum phosphorus levels into four quartiles, utilizing the bottom quartile as the reference group. The studies were then weighted using the inverse variance method. The I2 index and Q test were used at the α-level error of less than 10% significance to check the test heterogeneity of the included studies. Then, the random-effects model was used for heterogeneous data analysis. All data were entered in the STATA Version 14.
Risk of bias
Begg’s funnel plots and Egger test was selected to evaluate the potential publication bias in the data, and the P values of below 0.05 were considered to be valid for heterogeneity.
Results
Our study included 11 original studies1,9,11,13-20 evaluating the association between serum phosphate levels and mortality rates in pre-dialysis CKD and dialysis patients [Table 1]. The process of selecting studies is illustrated in Figure 1. In total, 297 studies were found through a systematic search of the literature. Then, after removing 84 studies due to duplication, 213 studies were included for the initial screen. Following a review of titles and abstracts, a total of 177 articles were excluded due to incompatibility with the inclusion criteria, leaving 36 for further examination. Finally, 14 articles were included, of which 11 qualified for meta-analyses. All studies were conducted between 2005 to 2021. The total sample size was 60,022, with a mean age of 62.46 (59.26–65.66) years. The pooled sample had a 65.34% male and 34.66% female gender distribution. The prevalence of diabetes was also reported in 44.44% of the total sample size. The duration of follow-up varied across the studies, but the mean follow-up duration among the studies was 47.16 months. Among 60,022 participants, 15,809 deaths were observed with an average mortality event of 14.83%. The average serum phosphate level and the average GFR were determined to be 4.24 mg/dL and 46.68 mL/min, respectively. The outcomes of our meta-analysis revealed the overall estimate of unadjusted HR of all-cause mortality per 1 mg/dL rise in the concentration of serum phosphate using the random-effects model in pre-dialysis CKD, and dialysis patients were 1.33 (95% CI: 0.97, 1.82, I2 = 99.1%, P < 0.001). After accounting for adjustments, every 1 mg/dL increase in serum phosphate level was associated with a 27% elevated risk of mortality (1.27 [95% CI: 1.15, 1.39, I2 = 75.4%, P < 0.001]) [Figure 2]. According to Begg’s funnel plots and Egger’s test, there is no significant publication bias among the included studies [Figure 3].
| Author name | Country | Year | CKD Stage | Sample size | Mean age ± SD | Serum P level (%) | Mortality events (%) | DM (%) | Mean GFR | Follow-up Duration (y) |
|---|---|---|---|---|---|---|---|---|---|---|
| Eddington9 | UK | 2010 | 1 to 5 | 1203 | 64±14 | 3.72 | 271 (%8.8) | 32 | 32 | 2.9 |
| Kestenbaum13 | USA | 2005 | 1 to 5 | 6730 | 71.2 | 3.5 | 1133 (%36.7) | 23 | 47.2 | 2.1 |
| Menon11 | USA | 2005 | 3 to 4 | 840 | 52 | 3.8 | 208 (%6.7) | 47 | 33 | 10 |
| Smith14 | USA | 2010 | 3 to 5 | 2122 | 68±13.6 | 3.61 | 625 (%20.2) | 29 | 47.8 | 5 |
| Voormolen15 | Netherland | 2007 | 2 to 4 | 448 | 60±15 | 4.71 | 30 (%1) | 3.8 | 13 | 1.5 |
| Kovesdy16 | USA | 2010 | 1 to 5 | 713 | 70±10 | 3.9 | 244 (%7.2) | 58 | 37 | 1.5 |
| Lee1 | Korea | 2007 | 5 | 3226 | 58.1±13.4 | 3.5 | 322 (%10) | 47.1 | 36 | 1.7 |
| Merhi17 | Canada | 2007 | Transplant | 3138 | 51.6±9.4 | 3.07 | 659 (%21) | 39 | 49 | 4 |
| Tiong18 | Australia | 2021 | 2 to 5 | 31989 | 60.6±14.9 | 2.97 | 12317 (%38.5) | 55 | 5.1 | |
| Wang19 | China | 2019 | 2 to 5 | 796 | 63.51±14.18 | 4.54 | 33.73 | 27.4 | 7 | |
| Lamina20 | Germany | 2020 | Dialysis | 8817 | 64.3±14.8 | 2.8 | 3100 (%35.2) | 36.1 | 26.4 | 8 |
CKD: Chronic kidney disease, GFR: Glomerular filtration rate, DM: Diabetes mellitus
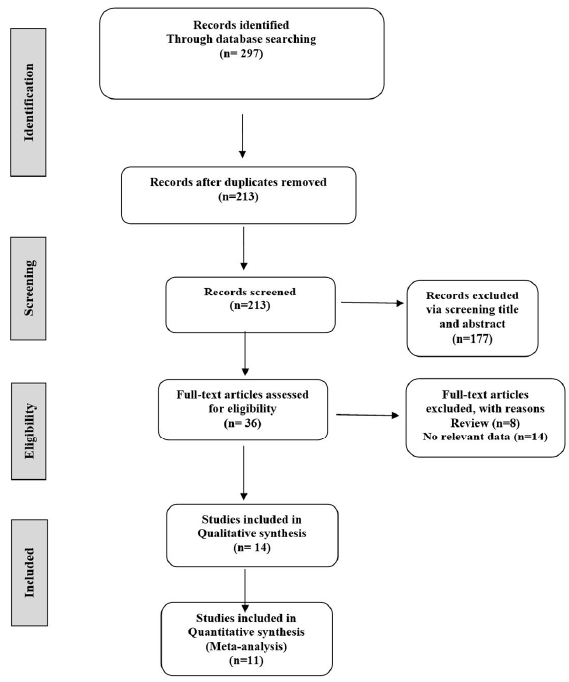
- Flow diagram of the study design process.
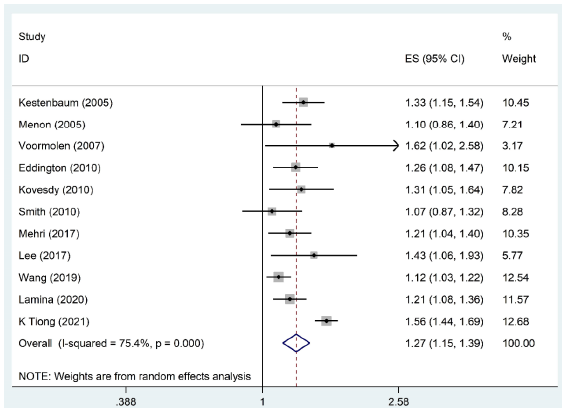
- Forest plot pooling odds ratios of the included studies in meta-analysis. ES: effect size, CI: confidence interval.
- Begg’s funnel plots and Egger’s test for publication bias among the included studies. s.e.: standard error.
The quality of included articles was evaluated in three main domains (including selection, comparability, and outcome) using NOS. The result of the quality assessment revealed that four studies had moderate quality and the remaining seven articles had high quality [Table 2].
| Author’s name | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | ||
| Eddington9 | * | * | * | * | * | 5 | |||
| Kestenbaum13 | * | ** | * | ** | - | 6 | |||
| Menon11 | * | * | * | ** | * | * | * | 8 | |
| Smith14 | * | * | * | * | * | * | * | 7 | |
| Voormolen15 | * | * | * | * | ** | * | - | 7 | |
| Kovesdy16 | * | * | * | * | ** | * | 7 | ||
| Lee1 | * | * | * | ** | * | 6 | |||
| Merhi17 | * | * | * | * | ** | * | 7 | ||
| Tiong18 | * | * | * | * | * | 5 | |||
| Wang19 | * | * | * | * | * | * | * | 7 | |
| Lamina20 | * | * | ** | * | * | * | 7 | ||
* and ** denote the score of each study in each part.
Discussion
The results of previous studies on the correlation between serum phosphate levels and mortality rates in pre-dialysis CKD and maintenance dialysis patients were contradictory. Numerous researchers have identified an inverse causal correlation: high serum phosphate levels cause proteinuria, which increases cardiovascular risk.8 Few studies have found a link between low serum phosphate and crude mortality as well as cause-specific mortality such as infection.1
The present meta-analysis was conducted to examine the correlation between serum phosphate levels and mortality rates in pre-dialysis CKD and maintenance dialysis patients, which demonstrated the linear relationship between serum phosphate levels and mortality rates in these patients. Our study suggests that after adjustment, each 1 mg/dL rise in the serum phosphate level corresponded to a 27% higher likelihood of mortality.
The previous three well-designed meta-analyses10,21,22 assumed the linear association between the concentration of serum phosphate and death rates among individuals with CKD. One of the meta-analysis studies conducted by Da et al. (2015) reported that each 1 mg/dL rise in concentration of serum phosphorus was associated with a 20% elevated likelihood of mortality.10 The difference between the Da et al.’s10 study and this study was that the study population in Da et al.10 was non-dialysis individuals with CKD (individuals who had received transplants were not included), but in our study, transplant recipients were also included in the study.
In a separate meta-analysis conducted by Natoli et al.,23 it was only verified that elevated phosphorus levels in dialysis patients, compared to the reference levels, were linked to a 20% higher risk of mortality. There was no substantial association found between relatively low phosphorus levels and death rates. These results suggested the idea that low levels of phosphorus increase all-cause death risk in individuals receiving dialysis. Natoli et al.’s meta-analysis showed that the association of phosphate with a death rate in pre-dialysis and dialysis CKD patients seems to be linear.23
Analyses conducted within specific subgroups demonstrated the impact of extremely high and extremely low phosphorus levels on the risk of all-cause mortality. It remains essential to conduct further investigations to determine if abnormal phosphorus levels have comparable effects on hemodialysis and peritoneal dialysis individuals.
The exact mechanisms by which abnormal phosphorus levels result in mortality are not understood. Phosphorus accumulation due to impaired kidney excretion may be one cause.24 Cardiovascular disease (CVD) is the most common cause of mortality in individuals receiving dialysis, and vascular calcification is an established risk factor. Vascular calcification correlates with the decline in renal function and reaches its peak in CKD.
Although dialysis therapy is progressing constantly, the mortality rate remains excessively elevated. The reason might be a disruption in mineral metabolism, like serum phosphate levels. In addition, the process of osteogenesis in smooth muscle cells could potentially affect the risk of vascular calcification.25
Several limitations ought to be taken into account. Initially, the outcomes of this meta-analysis were derived from a sole measurement of serum phosphate concentration taken at the baseline. Conducting a time average blood phosphate concentration examination will offer additional verification of the connection between irregular phosphate levels and all-cause deaths. Second, the circulating level of phosphate is controlled by vitamin D and FGF-23. The inappropriate adjustment of these two regulators may exaggerate the likelihood of risk. Third, this study was unable to establish the optimal cutoff values for phosphate that would lead to improved survival on dialysis therapy. Fourth, in our study, the relationship between phosphate level and the type of dialysis was not investigated. Finally, due to the limited number of studies, we were unable to conduct analysis based on the stage of CKD, which we presume would yield different results.
Conclusion
This meta-analysis highlights a linear association between serum phosphorus levels and mortality rates in pre-dialysis CKD and dialysis patients. Previous meta-analyses using relatively lower levels of phosphorus as a point of comparison might have undervalued the strength of an association.
Conflicts of interest
There are no conflicts of interest.
References
- Low serum phosphate as an independent predictor of increased infection-related mortality in dialysis patients: A prospective multicenter cohort study. PLoS One. 2017;12:e0185853.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296-309.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for CKD progression: Overview of findings from the CRIC study. Clin J Am Soc Nephrol. 2021;16:648-59.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for progressive chronic kidney disease. J Am Soc Nephrol. 2003;14:S65-70.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of interactions between proteinuria, activity of fibroblast growth factor 23 and serum phosphate on renal progression in patients with chronic kidney disease: A result from the Korean cohort study for outcome in patients with chronic kidney disease study. Nephrol Dial Transplant. 2020;35:438-46.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury following transcatheter aortic valve implantation: Incidence, predictors and clinical outcome. Int J Cardiol. 2013;168:1034-40.
- [CrossRef] [PubMed] [Google Scholar]
- Renal resistive index in chronic kidney disease patients: Possible determinants and risk profile. PLoS One. 2020;15:e0230020.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effects of VARC-defined acute kidney injury after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. Euro Intervention. 2012;8:563-70.
- [CrossRef] [PubMed] [Google Scholar]
- Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:2251-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum phosphorus and progression of CKD and mortality: A meta-analysis of cohort studies. Am J Kidney Dis. 2015;66:258-65.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis. 2005;46:455-63.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34.
- [CrossRef] [PubMed] [Google Scholar]
- Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520-8.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes predicted by phosphorous in chronic kidney disease: A retrospective CKD-inception cohort study. Nephrol Dial Transplant. 2010;25:166-74.
- [CrossRef] [PubMed] [Google Scholar]
- High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909-16.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clinical nephrology. 2010;73:268-75.
- [CrossRef] [PubMed] [Google Scholar]
- Serum phosphorus and risk of cardiovascular disease, all-cause mortality, or graft failure in kidney transplant recipients: An ancillary study of the FAVORIT trial cohort. Am J Kidney Dis. 2017;70:377-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum phosphate and mortality in incident dialysis patients in Australia and New Zealand. Nephrol. 2021;26:814-23.
- [CrossRef] [Google Scholar]
- The relationship and threshold of serum phosphate with regard to the 28-day mortality risk in sepsis patients undergoing continuous renal replacement therapy. J Int Med Res. 2020;48:0300060519831896.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association of changes in bone mineral parameters with mortality in haemodialysis patients: Insights from the ARO cohort. Nephrol Dial Transplant. 2020;35:478-87.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. Jama. 2011;305:1119-27.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease—a systemic review and meta-analysis. London, England: SAGE Publications Sage UK; 2011.
- Is there an association between elevated or low serum levels of phosphorus, parathyroid hormone, and calcium and mortality in patients with end stage renal disease? A meta-analysis. BMC Nephrol. 2013;14:1-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724-31.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int J Cardiol. 2017;238:151-8.
- [CrossRef] [PubMed] [Google Scholar]







