Translate this page into:
The Burden of Peripheral Neuropathy in Nondiabetic Chronic Kidney Disease and the Role of Ghrelin Isoforms in its Development
Current affiliation of Madumathy Ramachandran is “Department of Physiology, Saveetha Medical College * Hospital, Chennai, India”
Address for correspondence: Dr. Velkumary Subramanian, Academic Centre, JIPMER), Pondicherry, India. E-mail: velkumary@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Peripheral neuropathy is one of the most common complications in chronic kidney disease (CKD). The neuroprotective role of ghrelin is being explored recently. Here we aim to determine the burden of neuropathy in nondiabetic CKD and to find the association of peripheral nerve function with plasma ghrelin levels in these patients.
Methods:
This was a cross-sectional study conducted in nondiabetic CKD patients on conservative management to determine the magnitude of neuropathy. The association of ghrelin isoforms with nerve functions was assessed between three groups, namely CKD with neuropathy, CKD without neuropathy, and healthy volunteers, with 20 participants in each group.
Results:
The proportion of neuropathy in nondiabetic CKD was 78% (n = 78), of which 51% (n = 40) were asymptomatic. Des acyl ghrelin (DAG) and total ghrelin (TG) levels were 1545.5 ± 487.4 and 1567.4 ± 485.3 pg/mL, respectively, in CKD patients with neuropathy and were found to be elevated compared to those without neuropathy, who had 1000.4 ± 264.2 and 1019.7 ± 264.3 pg/mL of DAG and TG, respectively (P < 0.001). Assessment of correlation between nerve conduction parameters and DAG levels showed positive correlation between DAG levels and common peroneal latency (r = 0.69; P < 0.01), median sensory latency (r = 0.45; P < 0.05), and sural latency (r = 0.51; P < 0.05). We found negative correlation between median velocity (r =−0.56; P < 0.05), common peroneal velocity (r = −0.64; P < 0.01), median sensory velocity (r =−0.49; P < 0.05), and sural velocity (r = −0.54; P < 0.05). There was no statistically significant difference in acyl ghrelin levels among the groups.
Conclusion:
The prevalence of peripheral neuropathy in CKD is significantly higher with almost half of them being asymptomatic. Impaired renal clearance in CKD leads to the accumulation of DAG, which subsequently inhibits the neuroprotective functions of AG leading to neuropathy in CKD.
Keywords
Acyl ghrelin
chronic kidney disease
des acyl ghrelin
nerve conduction study
peripheral neuropathy
Introduction
Chronic kidney disease (CKD) has shown a significant rise globally over the past three decades, with a current worldwide prevalence of 13%.[12] Peripheral neuropathy is one of the most common but often overlooked complications in CKD.[3] The development of neuropathy can be attributed to uremia, inflammation, oxidative stress, and more recently to elevated potassium levels.[4] Uremic neuropathy is generally a distal sensorimotor neuropathy that predominantly affects the lower limbs.
Ghrelin, once thought to be an orexigenic hormone, has now been shown to have a neuroprotective role in various CNS disorders. Ghrelin is made of 28-amino acid peptides and is mainly secreted by the oxyntic cells of the stomach.[5] Ghrelin exists in two isoforms in circulation: acyl ghrelin (AG) and des acyl ghrelin (DAG). AG is the active form that contributes to 10% of total circulating ghrelin.[6] AG is formed from the octanoylation of proghrelin by the enzyme ghrelin O-acyltransferase.[7] It acts on growth hormone secretagogue receptor 1a to exert its action.[8] AG is metabolized to DAG where the acyl group is removed by the Butyrylcholinesterase enzyme.[9] It contributes to more than 90% of total ghrelin (TG). The receptor on which DAG acts is yet to be identified. The DAG is metabolized and eliminated by the kidney.[10] The well-known effects of AG are appetite stimulation, increased body weight, growth hormone release, increased gastrointestinal motility, and stimulation of energy production.[11] Though DAG was initially thought to be an inert substance, recent evidence has shown that it plays a significant role in opposing various actions of AG.[12]
Studies conducted on various cognitive disorders have documented the neuroprotective role of AG.[13] In an experimental mouse model of Parkinson's disease, ghrelin supplementation decreased the production of reactive oxygen species and prevented neural degeneration.[14] A study conducted by Tsuchimochi. et al.[15] showed that ghrelin supplementation prevented neuropathy in diabetes-induced rodents.
Neuropathy in CKD is the most distressing complication as it leads to the most disabling symptoms that affect the quality of life. Thus, in this study, we aimed to determine the burden of peripheral neuropathy in nondiabetic CKD (NDCKD) patients on conservative management. Moreover, understanding various plausible mechanisms leading to neuropathy is important to design specific therapies for its prevention or treatment. Given the role of AG in neuroprotection, we further aimed to determine the association between the ghrelin isoform levels and the nerve functions in CKD patients.
Methodology
Sample size calculation
Considering the expected percentage of peripheral neuropathy in adult nondiabetic stage 3–5 CKD patients on conservative therapy as 65%, absolute precision of 10%, α error of 5% (95% significance level), and attrition of 10%, a total of 100 individuals with nondiabetic stage 3–5 CKD patients on conservative therapy was required for the assessment of peripheral nerve function. The sample size was calculated using Open-epi software version 3.
Assuming the mean (SD) of TG levels as 275 ± 18, 1000 ± 725 and 1500 ± 800 pg/mL among healthy volunteers, CKD without peripheral neuropathy, and with peripheral neuropathy, respectively, power of 90%, α error of 5%, attrition of 10%, we required 20 individuals in each group for the assessment of plasma ghrelin levels. The sample size was calculated using G power software version 3.1.9.2.[16]
Procedure
We recruited 100 NDCKD patients in stages 3–5, aged between 18 and 50 years from the Nephrology Outpatient Department, JIPMER, Puducherry to assess the proportion of neuropathy. For further comparison of ghrelin levels and to determine the association of ghrelin levels with nerve conduction parameters, we took three groups: CKD patients with neuropathy (n = 20), CKD patients without neuropathy (n = 20), and healthy volunteers as control group (n = 20). We commenced our study after obtaining approval from the institute's ethics committee. The study was conducted in the Electrophysiology Laboratory, Department of Physiology, JIPMER after obtaining written informed consent from all the subjects.
CKD patients with diabetes, those patients on dialysis treatment, and those with peripheral neuropathy due to other causes such as alcoholism, vitamin B12 deficiency, autoimmune disorders, and malignancy were excluded. We also excluded patients with abnormal BMI of <17.5 and ≥23 kg/m2 because the ghrelin levels were altered in them.
The subjects were asked to report after overnight fasting to maintain uniformity in ghrelin level assessment, and 5 mL of blood was collected for ghrelin isoform measurement. Anthropometric measurements such as weight, height, and BMI were taken. Clinical evaluation and nerve conduction study were performed.
On clinical evaluation, history pertaining to neuropathy such as sensory symptoms (pins, needles, numbness) and motor symptoms (weakness) were collected followed by neurological examination such as pinprick sensation, vibration, power, and deep tendon reflexes. The nerve conduction study was done using the NIHON KOHDEN-NEURO PACK EP/EMG machine, and Neuropack M1 (Model: MEB 9200 K) was used for the nerve conduction study.
To determine the severity of neuropathy, the total neuropathy score (TNS) was used, which is based on symptom, sign, and nerve conduction parameters. Scores were given from 1 to 4 for each component of TNS. Scores less than 10 were categorized as mild neuropathy, scores between 10 and 19 were considered moderate, and scores more than or equal to 20 were considered severe neuropathy.[17]
Motor Conduction Study
Motor nerves such as the median nerve, common peroneal nerve, and tibial nerve were assessed bilaterally. The nerve was stimulated at two points (distal and proximal points) to obtain the compound muscle action potential (CMAP). The active electrode was placed on the motor point of the muscle belly, the reference electrode was placed 4 cm distal to the active electrode, and the ground electrode was placed in between the stimulating and the recording electrode. Silver chloride surface electrodes were used for recording, and a constant current stimulator was used for stimulation of the nerve. The current was given at the rate of 1 Hz for a duration of 0.2 ms. The distal stimulation was done 8 cm proximal to the active electrode. The proximal stimulation point is specific for each nerve. The nerve was stimulated to 20% supramaximal stimulus to obtain the CMAP.[18]
Sensory conduction study
Sensory nerves such as the median nerve, superficial peroneal nerve, and sural nerve were assessed bilaterally. The sensory conduction study was performed antidromically. Ring electrodes were used for recording for upper limbs (digits), disc electrodes were used for lower limbs, and plate electrode was used as the ground. The stimulation point was 14 cm proximal to the active electrode. The current was increased until the action potential reached its maximum amplitude, after which the potential was averaged 10 times at the same current to obtain the sensory nerve action potential (SNAP).[18]
The latency, amplitude, and velocity obtained were compared with the normative data. The type of neuropathy was determined based on the following criteria. The patients were diagnosed to have axonal neuropathy if there was a decrease in amplitude with normal or slightly decreased conduction velocity but not <75% of the lower limit of normal and normal or slightly prolonged distal latency but not >130% of the upper limit of normal. Patients were classified to have demyelinating neuropathy if the amplitude was normal with a marked decrease in conduction velocity of <75% of the lower limit of normal and prolonged distal latency >130% of the upper limit of normal. Patients were classified as mixed neuropathy if features of both axonal neuropathy and demyelinating neuropathy were present.[18]
Assessment of ghrelin levels
Under aseptic precautions, 5 mL of blood was collected in an EDTA-coated container. Plasma separation was done within 30 min of blood collection by cold centrifugation at 2°C–8°C for 15 min at 2000–3000 RPM. Separated plasma was stored in Eppendorf tubes at − 80°C. Sandwich ELISA technique was used for measuring the levels of total, AG, and DAG.
Diagnostic kit from Bioassay Technology Laboratory, Shanghai, China was used with a sensitivity of 0.01 ng/mL and an intra-assay coefficient of variation (CV) of <8%, and inter-assay CV was <10% for TG. The sensitivity of the AG kit was 0.024 ng/mL and that of the DAG kit was 0.27 ng/L.
Statistical analysis
Data were analyzed using SPSS version 20. Patient characteristics were summarized as frequencies and percentages for categorical variables and as mean ± SD for continuous variables. CKD with peripheral neuropathy was summarized as the proportion with a 95% confidence interval. Plasma ghrelin levels in each of the three groups were summarized as mean ± SD, and a comparison of the mean among three groups was done by one-way ANOVA. Correlation between plasma ghrelin and peripheral nerve function was summarized as Pearson correlation coefficient. P < 0.05 was considered statistically significant.
Results
We recruited 100 NDCKD patients in stages 3–5 on conservative management, aged between 18 and 50 years. There were 35 patients in stage 3, 35 patients in stage 4, and 30 patients in stage 5. The mean age of participants in our study was 41.8 ± 7.8 years, with 88 males and 12 females. The mean serum creatinine was 3.2 ± 1.09 mg/dL, and blood urea was 66.98 ± 30.09 mg/dL. The mean duration of CKD was 3.07 ± 1.07 years. There were 47 patients with a duration of less than 2 years and 53 patients with a duration of more than 2 years [Table 1].
| Variable | Mean±SD |
|---|---|
| Demographics | |
| Age (yr) | 41.8±7.8 |
| Duration of CKD (yr) | 3.07±1.07 |
| Gender | n (%) |
| Males | 88 (88) |
| Females | 12 (12) |
| Stages | n (%) |
| Stage 3 | 35 (35) |
| Stage 4 | 35 (35) |
| Stage 5 | 30 (30) |
| Laboratory Values | |
| S. Urea (mg/dL) | 66.98±30.09 |
| S. Creatinine (mg/dL) | 3.2±1.09 |
| S. Sodium (mg/dL) | 140.2±5.3 |
| S. Potassium (mg/dL) | 4.46±1.7 |
| S. Chloride (mg/dL) | 104.5±2.4 |
| Random Blood sugar (mg/dL) | 88±10 |
SD: Standard deviation
Neuropathy proportion
The proportion of peripheral neuropathy among 100 NDCKD patients was 78 (78%), of which 38 (49%) had manifested with clinical features of neuropathy and 40 (51%) were found to have subclinical or asymptomatic neuropathy. Severity was assessed based on the TNS, 42 (54%) were found to have mild, 26 (33%) had moderate, and 10 (13%) had severe neuropathy [Figure 1]. Furthermore, 25 (71%) patients were diagnosed to have neuropathy in stage 3, 28 (88%) patients in stage 4, and 25 (88%) patients in stage 5. Asymptomatic or subclinical neuropathy was most common in patients with stage 3 CKD with 22 (88%), patients followed by 13 (46%) patients in stage 4 and five (20%) patients in stage 5 [Figure 2]. Prevalence of neuropathy was more common in patients with CKD duration of >2 years, with 47 (60%) patients compared to those with CKD duration <2 years with 31 (40%) patients. Sensorimotor type of neuropathy was the most common type with 48 (61%) being affected, followed by sensory type with 24 (31%) and motor type with 6 (8%) patients. Out of 38 patients who presented with clinical features of neuropathy, the most common symptom was sensory symptoms with 32 (84%) patients, followed by motor symptoms with 3 (7%) patients. The most common examination finding was decreased vibration with 32 (84%) patients affected, followed by altered deep tendon reflex with 24 (63%) patients. We had 56 (72%) patients with mixed neuropathy, followed by 15 (19%) patients with pure axonal type and seven (9%) patients with pure demyelinating type of neuropathy [Figure 3]. Among sensory nerves, the most common nerve affected in our study was the sural nerve, followed by the superficial peroneal nerve and median nerve. Among motor nerves, the most common nerve affected was the common peroneal nerve, followed by the posterior tibial nerve and median nerve.

- Proportion of severity of neuropathy based on total neuropathy score among CKD patients (n = 100). We found neuropathy in 78% of CKD patients, of which 42% had mild, 26% had moderate, and 10% had severe neuropathy.
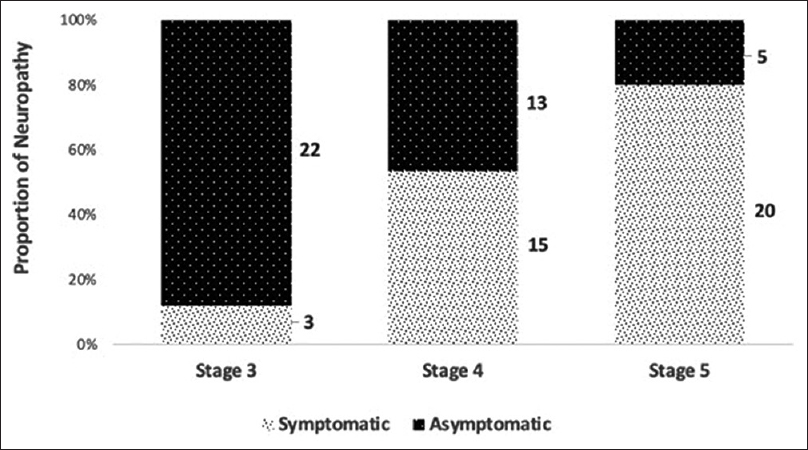
- Proportion of neuropathy based on the stage of CKD (n = 78). Patients in stage 3 were predominantly asymptomatic as compared to those in stage 5.
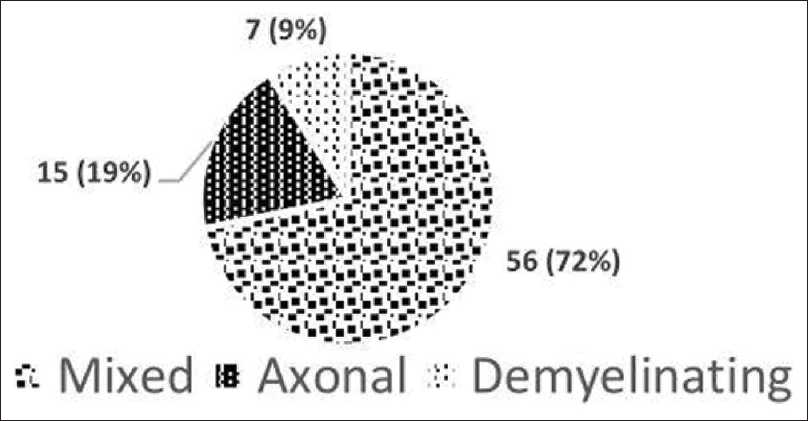
- Type of neuropathy among nondiabetic CKD patients (n = 78). Mixed neuropathy was most common followed by the pure axonal type and pure demyelinating type of neuropathy.
Patient Characteristics and Biochemical parameters of study participants for ghrelin assessment
A comparison of anthropometric and biochemical parameters between groups is presented in Table 2. Groups were comparable based on age, BMI, and RBS, which were within the normal range in all the groups, and there was no significant difference between the groups.
| Parameter | CKD with neuropathy Mean±SD (n=20) | CKD without neuropathy Mean±SD (n=20) | Healthy volunteers Mean±SD (n=20) | P |
|---|---|---|---|---|
| Age (yrs) | 40.2±8.1 | 45.65±6.45 | 41.9±9.1 | 0.105 |
| BMI (kg/m2) | 22.1±1.4 | 22.5±0.9 | 22.7±0.8 | 0.257 |
| S. Urea (mg/dL) | 66.0±28.1### | 52.0±13.4## | 13.2±2.8 | 0.000 |
| S. Creatinine (mg/dl) | 3.9±0.5### $$$ | 2.6±1.1### | 0.8±0.1$$$ | 0.000 |
| S. Uric acid (mg/dl) | 7.6±1.8### | 7.3±1.8### | 4.5±0.6 | 0.000 |
| S. Sodium (mEq/L) | 137.2±3.3 | 137.3±2.2 | 138.8±1.8 | 0.106 |
| S. Potassium (mEq/L) | 4.46±0.7# | 4.1±0.5 | 4.00±0.0 | 0.008 |
| S. Chloride (mEq/L) | 103.5±3.4# | 104.9±3.5## | 101.5±2.6 | 0.005 |
| Random Blood Sugar (mg/dl) | 97.0±9.8 | 100.6±14.5 | 94.2±3.2 | 0.124 |
Data analyzed by one-way ANOVA test; #shows comparison with Healthy volunteers, $ shows comparison with CKD without neuropathy; # P<0.05 # #P<0.01 ###P<0.001 $ P<0.05 $$ P<0.01 $$$ P<0.01; P value less than 0.005 is considered statistically significant; SD Standard deviation.
Comparison of ghrelin levels between groups
On comparison of mean ghrelin isoform levels between the groups, the TG levels and DAG showed a significant difference between the three groups (P < 0.001), whereas no significant difference was found in AG levels [Table 3].
| Parameter | CKD with neuropathy Mean±SD (n=20) | CKD without neuropathy Mean±SD (n=20) | Healthy volunteers Mean±SD (n=20) | P |
|---|---|---|---|---|
| Acyl ghrelin (pg/mL) | 21.8±7.1 | 19.6±3.8 | 20.6±4.4 | 0.415 |
| Des acyl ghrelin (pg/mL) | 1545.5±487.4###$$$ | 1000.4±264.2## | 614.5±207.6 | 0.000 |
| Total ghrelin (pg/mL) | 1567.4±485.3###$$$ | 1019.7±264.3## | 637.6±208.3 | 0.000 |
Data analyzed by one-way ANOVA test; #shows comparison with healthy volunteers, $shows comparison with CKD without neuropathy; #P<0.05 ##P<0.01 ###P<0.001 $P<0.05 $$P<0.01 $$$P<0.01;P<0.005 is considered statistically significant; SD: Standard deviation
Correlation of ghrelin levels with nerve conduction parameters between groups
On performing correlation test between nerve conduction parameters and DAG levels, we found positive correlation between TG levels and latency of motor nerves like common peroneal (r = 0.69; P < 0.01) and posterior tibial nerve (r = 0.65; P < 0.01). We observed positive correlation between TG levels and latency of sensory nerves like median (r = 0.45; P < 0.05) and sural nerve (r = 0.51; P < 0.05). We found negative correlation between TG levels and velocity of motor nerves like median (r = −0.56; P < 0.05), common peroneal (r = −0.64; P < 0.01), and posterior tibial nerve (r = −0.57; P < 0.01). The negative correlation was observed between TG levels and velocity of sensory nerves like median (r =−0.49; P < 0.05) and sural nerve (r = −0.54; P < 0.05) [Table 4].
| Nerve | Des acyl ghrelin | Total ghrelin | ||
|---|---|---|---|---|
| r | P | r | P | |
| Median (motor) | ||||
| Latency (ms) | 0.12 | 0.628 | 0.11 | 0.618 |
| Amplitude (mV) | −0.15 | 0.535 | −0.15 | 0.526 |
| Velocity (m/s) | −0.56 | 0.011 | −0.56 | 0.011 |
| Common peroneal | ||||
| Latency (ms) | 0.69 | 0.001 | 0.69 | 0.001 |
| Amplitude (mV) | −0.13 | 0.587 | −0.13 | 0.579 |
| Velocity (m/s) | −0.64 | 0.002 | −0.65 | 0.002 |
| Posterior tibial | ||||
| Latency (ms) | 0.64 | 0.002 | 0.64 | 0.002 |
| Amplitude (mV) | −0.36 | 0.114 | −0.36 | 0.11 |
| Velocity (m/s) | −0.57 | 0.007 | −0.58 | 0.008 |
| Median (sensory) | ||||
| Latency (ms) | 0.45 | 0.045 | 0.46 | 0.043 |
| Amplitude (mV) | −0.04 | 0.858 | −0.04 | 0.857 |
| Velocity (m/s) | −0.49 | 0.025 | −0.50 | 0.024 |
| Superficial peroneal | ||||
| Latency (ms) | 0.23 | 0.318 | 0.24 | 0.314 |
| Amplitude (mV) | −0.13 | 0.579 | −0.13 | 0.573 |
| Velocity (m/s) | −0.44 | 0.051 | −0.45 | 0.048 |
| Sural | ||||
| Latency (ms) | 0.51 | 0.023 | 0.50 | 0.022 |
| Amplitude (mV) | −0.29 | 0.203 | −0.30 | 0.199 |
| Velocity (m/s) | −0.54 | 0.014 | −0.54 | 0.013 |
Data analyzed by Pearson correlation; r: correlation coefficient; P<0.005 is considered statistically significant
On performing a correlation test between nerve conduction parameters and TG levels, we found positive correlation between TG levels and latency of motor nerves like common peroneal (r = 0.69; P < 0.01) and posterior tibial nerve (r = 0.65; P < 0.01). We observed positive correlation between TG levels and latency of sensory nerves like median (r = 0.46; P < 0.05), sural nerve (r = 0.50; P < 0.05). We found negative correlation with between TG levels and velocity of motor nerves like median (r = −0.56; P < 0.05), common peroneal (r = −0.65; P < 0.01) and posterior tibial nerve (r = −0.58; P < 0.01). The negative correlation was observed between TG levels and velocity of sensory nerves like median (r = −0.50; P < 0.05) and sural nerve (r = −0.54; P < 0.05) in the sensory conduction study [Table 4].
Discussion
CKD induces systemic inflammation that affects various organs, including the nervous system. Peripheral neuropathy is one of the most common neurological complications in CKD but often goes unnoticed in clinical practice until the patient presents with disabling symptoms.[3] In our study, we found a significantly higher proportion (78%) of peripheral neuropathy among NDCKD patients, of which almost half of the patients (51%) were asymptomatic and were diagnosed based on an abnormality in the nerve conduction study. Subclinical neuropathy was more common in the early stages. A mixed type of neuropathy was more common, followed by pure axonal and pure demyelinating types of neuropathy. The most common nerves involved in our study were sural and superficial peroneal followed by median nerves among sensory nerves. Among the motor nerves, the common peroneal nerve was most commonly affected, followed by the posterior tibial and median nerves. This finding proves the length-dependent neuropathy pattern as lower limb nerves are most commonly involved compared to upper limb nerves.
A study conducted by Aggarwal et al.[19] in 2013 showed approximately 70% of CKD patients had neuropathy. These findings are in concordance with our study and indicate that there exists a high prevalence of peripheral neuropathy among CKD patients and a considerable number of patients have subclinical neuropathy, which is often unnoticed until diagnosed by electrophysiological study or worsened to the most disabling neuropathy. Sensorimotor neuropathy was most common, followed by pure sensory and pure motor type. This observation is contradictory to a few other studies where pure sensory type is documented to be the most common type in CKD. A study conducted by Mallipeddi et al.[20] in 2017 showed similar results to our study where the mixed pattern is a more common type of neuropathy in CKD patients.
Periodic assessment of nerve functions by nerve conduction study in CKD patients is the objective test for early detection of subclinical peripheral neuropathy and enrolment of early intervention in these patients, which, in turn, will improve their quality of life.
Ghrelin is an orexigenic hormone, but recent studies conducted on various cognitive disorders explored its neuroprotective property. In our study, the TG and DAG levels were found to be increased in CKD compared to healthy volunteers. Among CKD patients, TG and DAG levels were higher in CKD patients with neuropathy compared to those without neuropathy, and the differences were statistically significant. However, the levels of AG showed no difference among the groups. Gupta et. al. in their study findings showed elevated levels of DAG in CKD patients, which is in concordance with our study.[19] The levels of AG were reported to have conflicting results from various studies.[2122] Because the secretion of AG and its conversion to DAG is unaffected, the levels of AG were found to be normal in our study. However, the converted DAG starts to accumulate due to impaired renal clearance in these patients who have compromised renal function. As the TG level is a reflection of the DAG levels due to its 4:1 des acyl to acyl ratio,[923] we got elevated TG levels in CKD patients.
Systemic inflammation is a hallmark of CKD which leads to various complications, including neuropathy. AG exerts its anti-inflammatory action by inhibiting cytokines at various stages of the inflammatory process and thereby preventing apoptosis of neurons.[13] This is proved in a study where ghrelin administration decreased the levels of oxidative stress markers and circulating cytokines levels.[24] We assume that when the level of DAG increases to a certain extent as in CKD, it can induce neuropathy by suppressing the neuroprotective function of AG. This hypothesis is evidenced in our study findings, wherefirst we demonstrated a statistically significant increase in DAG levels in CKD patients with neuropathy compared to those without neuropathy. Second, we showed a correlation between elevated des acyl levels and worsening nerve conduction parameters. Several studies have shown that AG exerts neuroprotective properties through its antioxidant and anti-inflammatory effects.[2526] Recent studies have shown that DAG at abnormally higher levels can antagonize the effect of AG.[2728] Such an opposing effect is possible either due to the prevention of AG from binding to GHSR 1a or by binding to a yet unidentified DAG receptor.[25] The neuroprotective role of ghrelin has been documented by various studies. Ueno et al. demonstrated a significant improvement in motor conduction velocity in the posterior tibial nerve following short-term administration of ghrelin to patients with diabetic neuropathy.[29] An animal study conducted in diabetes-induced mice reported the prevention of neuropathy on ghrelin supplementation.[15] Ishii et al. showed prevention of intraepidermal nerve fiber loss by ghrelin supplementation in paclitaxel-induced neuropathy.[30]
Conclusion
Peripheral neuropathy in nondiabetic CKD is a huge burden with significantly higher prevalence. The majority of them were asymptomatic with subclinical neuropathy. In CKD, the secretion of AG and its conversion to DAG remains intact. However, the DAG accumulates due to impaired renal clearance. Hence, we presume that the elevated levels of DAG in circulation impede the neuroprotective action of AG, leading to neuropathy in CKD patients.
Financial support and sponsorship
Intramural funding, JIPMER, Puducherry, India. The grant number is JIP/Res/Intra-MD-MS/phs1/01/2017-18.
The funding organization played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I sincerely thank the technical staff who have extended their support throughout the duration of my project. I extend my deepest gratitude to the director of JIPMER for his support.
References
- Global prevalence of chronic kidney disease – A systematic review and meta-analysis. PLoS One. 2016;11:e0158765.
- [Google Scholar]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-33.
- [Google Scholar]
- A Clinical and electrophysiological study of peripheral neuropathies in predialysis chronic kidney disease patients and relation of severity of peripheral neuropathy with degree of renal failure. J Neurosci Rural Pract. 2017;8:516-24.
- [Google Scholar]
- Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis. 2016;5:204800401667768.
- [Google Scholar]
- Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-60.
- [Google Scholar]
- Ghrelin O -acyltransferase (GOAT), a specific enzyme that modifies ghrelin with a medium-chain fatty acid. J Biochem (Tokyo). 2016;160:189-94.
- [Google Scholar]
- Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426-57.
- [Google Scholar]
- The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. Eur J Endocrinol. 2013;168:821-8.
- [Google Scholar]
- Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol. 2002;13:2748-52.
- [Google Scholar]
- Ghrelin: Much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 2013:619-24.
- [Google Scholar]
- Des-acyl ghrelin: A metabolically active peptide. 2013. Endocrine Development. Basel: S. KARGER AG; :112-21. Available from: https://www.karger.com/Article/FullText/346059
- [Google Scholar]
- Acylated but not des-acyl ghrelin is neuroprotective in an MPTP mouse model of Parkinson's disease. J Neurochem. 2016;137:460-71.
- [Google Scholar]
- Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057-65.
- [Google Scholar]
- Ghrelin prevents the development of experimental diabetic neuropathy in rodents. Eur J Pharmacol. 2013;702:187-93.
- [Google Scholar]
- Effect of chronic kidney disease on circulating ghrelin concentrations. Ann Rom Soc Cell Biol. 2015;2:41-46.
- [Google Scholar]
- The utility of the Total Neuropathy Score as an instrument to assess neuropathy severity in chronic kidney disease: A validation study. Clin Neurophysiol. 2018;129:889-94.
- [Google Scholar]
- Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations (2nd). Philadelphia: Elsevier, Butterworth-Heinemann; 2005. p. :685.
- Evaluation of spectrum of peripheral neuropathy in predialysis patients with chronic kidney disease. Ren Fail. 2013;35:1323-9.
- [Google Scholar]
- A clinical and electrophysiological study of peripheral neuropathies in peritoneal dialysis patients: Our experience from rural South India. Saudi J Kidney Dis Transplant. 2018;29:1139-49.
- [Google Scholar]
- Association of plasma des-acyl ghrelin levels with CKD. Clin J Am Soc Nephrol. 2013;8:1098-105.
- [Google Scholar]
- Increased serum acylated ghrelin levels in patients with mild cognitive impairment. J Alzheimers Dis. 2017;61:545-52.
- [Google Scholar]
- Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980-7.
- [Google Scholar]
- Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148:148-59.
- [Google Scholar]
- The multifunctional and multi-system influence of Ghrelin in the treatment of diabetic and spinal cord injury induced neuropathy. Ann Neurosci. 2011;18:118-22.
- [Google Scholar]
- The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011;340:44-58.
- [Google Scholar]
- Mechanisms in endocrinology: Ghrelin: the differences between acyl- and des-acyl ghrelin. In: Eur J Endocrinol. Vol 167. 2012. p. :601-8.
- [Google Scholar]
- Ghrelin and des-acyl ghrelin. 2000. p. :909-13.
- Clinical application of ghrelin for diabetic peripheral neuropathy. Endocr J. 2017;64:S53-7.
- [Google Scholar]
- Ghrelin alleviates paclitaxel-induced peripheral neuropathy by reducing oxidative stress and enhancing mitochondrial anti-oxidant functions in mice. Eur J Pharmacol. 2018;819:35-42.
- [Google Scholar]







