Translate this page into:
The kidney in pregnancy: A journey of three decades
Address for correspondence: Dr. Jai Prakash, Department of Nephrology, Institute of Medical Sciences, Banaras Hindu University, Varanasi – 221 005, Uttar Pradesh, India. E-mail: jprakash53@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The spectrum of kidney disease occurring during pregnancy includes preeclampsia, hypertensive disorders of pregnancy, urinary tract infection, acute kidney injury, and renal cortical necrosis (RCN). Preeclampsia affects approximately 3–5% of pregnancies. We observed preeclampsia in 5.8% of pregnancies, and 2.38% of our preeclamptic women developed eclampsia. Severe preeclampsia and the eclampsia or hemolysis, elevated liver enzymes levels, and low platelets count (HELLP) syndrome accounted for about 40% of cases of acute kidney injury (AKI) in pregnancy. Preeclampsia/eclampsia was the cause of acute renal failure (ARF) in 38.3% of the cases. Preeclampsia was the most common (91.7%) cause of hypertension during pregnancy, and chronic hypertension was present in 8.3% of patients. We observed urinary tract infection (UTI) in 9% of pregnancies. Sepsis resulting from pyelonephritis can progress to endotoxic shock, disseminated intravascular coagulation, and AKI. The incidence of premature delivery and low birth weight is higher in women with UTI. The incidence of AKI in pregnancy with respect to total ARF cases has decreased over the last 30 years from 25% in 1980s to 5% in 2000s. Septic abortion-related ARF decreased from 9% to 3%. Prevention of unwanted pregnancy and avoidance of septic abortion are key to eliminate abortion-associated ARF in early pregnancy. The two most common causes of ARF in third trimester and postpartum periods were puerperal sepsis and preeclampsia/HELLP syndrome. Pregnancy-associated thrombotic thrombocytopenic purpura/hemolytic uremic syndrome and acute fatty liver of pregnancy were rare causes of ARF. Despite decreasing incidence, AKI remains a serious complication during pregnancy.
Keywords
Acute kidney injury
kidney disease
preeclampsia
pregnancy
renal cortical necrosis
Introduction
Pregnancy is characterized by a myriad of physiological changes, of which the emergence of a placenta and growing fetus is the most dramatic. Hypertension and/or renal disease occurring in the setting of pregnancy present a unique set of clinical challenges. There are several anatomical and physiological adaptations occurring in the kidney during the course of normal pregnancy [Table 1].[1] The knowledge of these physiological changes of pregnancy is of clinical importance. Serum creatinine and BUN of 1.0 mg/dl and 13 mg/dl, respectively, would be considered normal in a non-pregnant individual, but reflect renal impairment in a pregnant woman.

Preeclampsia, Eclampsia and Hemolysis, Elevated Liver Enzymes, and Low Platelets (HELLP) Syndrome
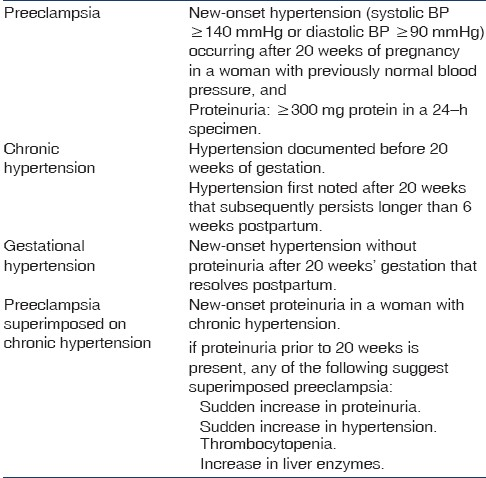
It is a pregnancy-specific systemic syndrome, characterized by the new onset of hypertension and proteinuria after 20 weeks of gestation. Preeclampsia affects approximately 3–7.5% of pregnancies worldwide.[23] The reported frequencies of preeclampsia–eclampsia in two studies were 5–7%.[45] Menon has noted incidence of preeclampsia between 7% and 9% in an Indian study.[6] The incidence of preeclampsia–eclampsia were 8.3% and 5.8%, respectively, in our studies[78] and thus our observation was similar to the other Indian study.[6] Eclampsia is defined as new-onset grand mal seizures during pregnancy or within 4 weeks postpartum in a woman with preeclampsia. It occurs in approximately 2% of preeclampsia cases in the United States.[9] We reported eclampsia in 2.38% of cases.[8] Up to one-third of eclampsia occurs postpartum, sometimes days to weeks after delivery.[10] Antepartum and postpartum eclampsia were reported in 72% and 28% of cases, respectively, and our observations were similar to other studies.[81011]
Renal manifestations of preeclampsia
Preeclampsia is a pregnancy-specific vascular endothelial disorder with varying degrees of multiple organ involvement, of which kidney bears the main crunch, being a highly vascular organ. In this section, spectra of kidney manifestations of preeclampsia are discussed, and they include: (i) hypertension, (ii) proteinuria, (iii) acute kidney injury (AKI), and (iv) postpartum recovery.
Hypertension
For the diagnosis of preeclampsia, hypertension is defined as a systolic blood pressure of 140 mmHg or higher or a diastolic blood pressure of 90 mmHg or higher after 20 weeks of gestation in a woman with previously normal blood pressure.[12] Hypertension should be confirmed by two separate measurements at least 4 h apart. Hypertension is a universal feature of preeclampsia. However, hypertension may be absent in a few patients who develop eclampsia or hemolysis, elevated liver enzymes levels, and low platelets count (HELLP) syndrome. The severity of hypertension in preeclampsia can vary widely, from mild hypertension to severe hypertension associated with headache and visual changes, resistant to multiple medications.
Proteinuria
Proteinuria is defined as urinary excretion of more than 300 mg of protein during 24-h collection. However, the urine protein-to-creatinine ratio is an excellent tool to estimate the degree of proteinuria during pregnancy, and has a strong correlation with 24-h urinary protein excretion.[1] The mean 24-h urinary protein excretion in our studies was 1.5±2.5 g, and similar observations were made by other workers.[13–15] Preeclampsia is the leading cause of nephrotic syndrome (NS) during pregnancy.[16] We reported NS in 11.3% of cases, and two patients had proteinuria of >5 g/day.[11] Proteinuria disappeared in all the patients with an average duration of 35.8 (21– 90) postpartum days. Persistent proteinuria and/or hypertension beyond 12 weeks postpartum is an indication for ruling out primary renal diseases. Renal histology in two such patients showed focal segmental sclerosis and mesangiocapillary glomerulonephritis in one each.[11] The patient is classified as having severe preeclampsia when proteinuria exceeds 5 g/day. However, heavy proteinuria alone is not an indication for urgent delivery. The traditional criteria to confirm a diagnosis of preeclampsia (new-onset hypertension and proteinuria after 20 weeks’ gestation) are appropriate to use for most healthy nulliparous women. However, hypertension or proteinuria might be absent in 10–15% of women who develop HELLP syndrome and 38% of those who develop eclampsia.[1718] This is known as atypical or non-proteinuric preeclampsia. In the absence of proteinuria, preeclampsia should be considered when hypertension is associated with persistent cerebral symptoms, epigastric pain, thrombocytopenia, and/or abnormal liver enzymes.
Acute kidney injury
Severe preeclampsia and the HELLP syndrome account for about 40% of cases of AKI in pregnancy.[19] Up to 20% of women with severe preeclampsia develop HELLP syndrome.[20–22] ARF occurs in approximately 1% of women with severe preeclampsia[19] and 3–15% of women with HELLP syndrome,[23–25] and renal failure can occur either antepartum or in the early postpartum period.[212225] ARF in pregnancy is detailed in Section 4 of this chapter.
Postpartum recovery
Generally, preeclampsia begins to remit soon after delivery of the fetus and placenta, and complete recovery is the rule. However, normalization of blood pressure and proteinuria takes day to weeks. Pre-eclampsia may have the following long-term consequences.[26]
-
Over 50% of women with preeclampsia develop hypertension.
-
The relative risks of subsequent ischemic heart disease, stroke, and cardiovascular mortality are more than double in women who have had preeclampsia.[26]
-
Preeclampsia has also been recently recognized as a major risk factor for chronic kidney disease (CKD).[27]
Hypertensive Disorders of Pregnancy
The definition and classification of hypertensive disorders of pregnancy is summarized in Table 2. These guidelines help to distinguish preeclampsia, chronic hypertension in pregnancy, gestational hypertension, and chronic hypertension with superimposed preeclampsia. Chronic hypertension is present in 3–5% of pregnancies and is more common with advanced maternal age, obesity, and black race.[28] The diagnosis of chronic hypertension in pregnancy is usually based on a documented history of hypertension prior to pregnancy or blood pressure above 140/90 mmHg prior to 20 weeks’ gestation. Hypertensive disorders may complicate about 3–10% of all pregnancies with variable incidence among different hospitals and countries.[2930] We observed hypertension in 5.38% of pregnant women, and preeclampsia was the dominant cause (91.7%).[78] Chronic hypertension accounted for only 8.3% of patients in our study.[6] Pregnant women with chronic hypertension have an increased risk of preeclampsia (21–25%), premature delivery (33–35%), intrauterine growth retardation (IUGR)(10–15%), placental abruption (1–3%), and perinatal mortality (4.5%).[31] Of 97 hypertensive patients, perinatal death and low birth weight occurred in 37.5% and 66.60% of deliveries, respectively, and live birth was seen in 62.5% of cases in our study.[6] Thus, hypertension during pregnancy is associated with adverse fetal outcomes and carries maternal mortality rate of 5.5%.[6] Gestational hypertension and preeclampsia are considered to be part of the same spectrum of illness. Gestational hypertension typically resolve postpartum. However, it can progress to overt preeclampsia in 10–25% of cases. We did not observe gestational hypertension in our study.[7]
UTI During Pregnancy
It is the most frequent renal problem encountered during gestation.[32] The prevalence of asymptomatic bacteriuria in pregnant women is similar (2–10%) to the non-pregnant population. However, it needs more aggressive management because of the high maternal mortality and morbidity associated with pyelonephritis. Untreated asymptomatic bacteriuria in pregnancy can progress to overt cystitis or acute pyelonephritis in up to 40% of patients.[33] Acute pyelonephritis is a serious complication, usually presenting at/or between 20 and 28 weeks of gestation with fever, loin pain, and dysuria. Sepsis resulting from pyelonephritis can progress to endotoxic shock, disseminated intravascular coagulation (DIC), and AKI. Asymptomatic bacteriuria has also been associated with an increased risk of premature delivery and low birth weight.[34] Treatment can improve perinatal morbidity and mortality.[35] Thus, early detection and prompt treatment of asymptomatic bacteriuria is warranted.[36] We observed UTI in 9% of pregnancies in our study. Third trimester was associated with highest number of UTI (11.9%), followed by second (7.5%) and first (5.7%). Approximately 35% of the culture-positive women were asymptomatic. Among the fetal complications, low birth weight babies were significantly more frequent in patients with UTI than in those without (P<0.05). In addition, intrauterine death, prematurity, and abortion were more frequent, though not significant (P>0.05), in culture-positive than the culture-negative women.[37]
Acute Kidney Injury in Pregnancy
Pregnancy-related acute kidney injury (PR-AKI) is rare and usually occurs in women with previously healthy kidneys. Despite converging trends, there are still huge differences in the incidence, causes, and outcome of PR-AKI in developed countries compared with developing countries.[3839] This is closely linked to environmental and socioeconomic conditions. With the liberalization of abortion laws and improved obstetric care, PR-AKI is now an uncommon occurrence in developed countries.[4041] Since the 1960s, the incidence of PR-AKI has decreased from 1/3,000 to 1/15,000 – 1/20,000 with respect to the total number of pregnancies. Similarly, the proportion of PR-AKI from total cases of AKI has fallen from 20–40% in the 1960s to 0–1% in the last decade.[41] In developing countries, PR-AKI has decreased in recent years, but still accounts for 5–20% of total AKI.[394243]
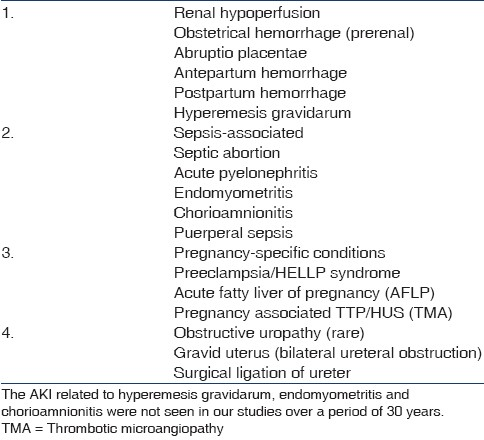
The incidence of AKI in pregnancy has dramatically decreased over the last 50 years, owing mainly to the decreased rate of septic abortion and improved obstetric care in developed countries.[404144] In contrast, septic abortion-related AKI is still high in some parts of developing countries.[45] The causes of AKI during pregnancy can be broadly categorized into prerenal, postrenal, and intrinsic renal injury, similar to AKI in the general adult population. Although, prerenal and ischemic causes of AKI are the most common, renal insufficiency can complicate several pregnancy-specific conditions [Table 3]. In particular, severe preeclampsia/HELLP syndrome, pregnancy-associated thrombotic thrombocytopenic purpura (TTP)/hemolytic uremic syndrome (HUS), and acute fatty liver of pregnancy (AFLP) are all frequently complicated by acute renal failure (ARF). In developing countries, the common contributing factors to PR-AKI are abruptio placentae, puerperal sepsis, septic abortion, and postpartum hemorrhage;[39424647] whereas in developed countries, the most common causes of PR-AKI are preeclampsia/HELLP syndrome, thrombotic microangiopathy (TTP/HUS) of pregnancy, sepsis, and hemorrhage by abruptio placentae.[40] Despite decreasing incidence, PR-AKI remains a serious complication, with 10–30% of patients progressing to end-stage renal disease.[4142]
Sepsis-associated AKI
Sepsis is frequently associated with hypotension, inflammation, DIC, HUS, and AKI. Most cases of sepsis occur during late pregnancy and the postpartum period. Early recognition and treatment of sepsis-associated PR-AKI may prevent complications. In an European study about 1% of all pregnant women required ICU admission, and 4% of them had severe sepsis or septic shock.[48] The most common infection was chorioamnionitis (40%) and endomyometritis (20%).[48] The common infections causing sepsis in pregnancy in developing countries are septic abortion, pyelonephritis, and postpartum fever.[4549] Puerperal sepsis is the most common cause of PR-AKI in our country.[434950] Septic abortion-related AKI has decreased in recent years. In the Chandigarh study, ARF due to septic abortion, which constituted 13.7% of total ARF during 1965–1974, had declined to 3.5% by 1981–1986. Post-abortal ARF decreased to 7% in 1992–2002 from 9% in 1982–1991 of total ARF cases.[39] However, despite declining trends in post-abortal ARF, it is still high in some parts of the country. The social stigma attached to abortion is probably the main cause of high incidence of septic abortions in our society. Despite legalization of abortion law, a good number of pregnant women seek abortion from untrained and unlicensed abortionists under unhygienic conditions.[47] Prevention of unwanted pregnancy and avoidance of septic abortion are key to eliminate abortion-associated ARF in early pregnancy. In addition, sepsis also accounts for the majority of maternal mortality in PR-ARF in developing countries.[4950]
Severe preeclampsia/HELLP syndrome
Severe preeclampsia and the HELLP syndrome account for about 40% of cases of AKI in pregnancy.[19] Preeclampsia/eclampsia/HELLP syndrome was the most common (38.3%) cause of ARF in late pregnancy in our study.[49] Acute renal failure occurs in approximately 1% of women with severe preeclampsia and 3–15% of women with HELLP syndrome; and renal failure can occur either antepartum or in early postpartum period.[22–25] The HELLP syndrome almost always occurs in the setting of preeclampsia, and is considered to be a form of severe preeclampsia. The characteristic features of HELLP syndrome include hemolysis (schistocytes on peripheral smear, anemia, increased LDH, and decreased haptoglobin), elevated liver enzymes, and low platelet count. The platelet count is a marker of the severity of disease and coincides with liver impairment.[20] Thrombocytopenia is often the first sign of HELLP syndrome; hence any patient with a significant drop in platelet count during the antenatal period should be suspected of the disease. When HELLP syndrome is complicated by AKI, the risk of perinatal death is higher (26%) and increases with the severity of the renal injury.[23] AKI most often develops in the setting of severe complications (placental abruption, DIC, postpartum bleeding or fetal death) and the likelihood of renal failure increase with the severity of HELLP syndrome.[82021] The exact prevalence of AKI in patients with HELLP syndrome is not well known. However, a large study estimated that AKI complicates 3% of severe HELLP syndrome.[51]
About 30-50% of patients with HELLP syndrome and AKI require dialysis.[2139] Renal function typically improves following delivery. Persistent renal dysfunction and/or need for long-term dialysis is unusual, but can occur in women with preexisting hypertension or CKD.[3940] Acute tubular necrosis is a nearly universal pathological lesion in patients with HELLP syndrome-associated AKI.[52–54] However, occasionally, RCN has been reported.[424955] Maternal mortality in AKI complicating HELLP syndrome was high (13%) in studies performed in the 1980s;[21] more recent reports suggest a much lower incidence.[2356]
Pregnancy-associated TTP/HUS
TTP and HUS are rare during pregnancy and postpartum period. HUS/TTP is characterized by thrombocytopenia, hemolysis, and variable organ dysfunction, which can include neurological changes and ARF.[57] Patients are believed to have TTP when neurological symptoms dominate and HUS when renal failure is the dominant presenting feature. TTP usually present prior to 24 weeks and HUS typically occurs near term or postpartum. However, pregnancy-associated TTP can occur in third trimester or during the postpartum period as well.[5859] TTP can develop de novo in pregnancy, or pregnancy can precipitate relapse in women with history of TTP.[60] Patients with congenital or familial TTP frequently have their first episode during pregnancy. Increasing concentration of procoagulant (factor VIIa, factor VIII, von Willebrand factor, and fibrinogen) factors, decreased fibrinolytic activity, and deficiency of von Willebrand factor-cleaving protease (ADAMTS 13) may contribute to development of TTP/HUS in susceptible individuals. In pregnancy-associated TTP, the deficiency of ADAMTS13 can be quite severe, with about 20% of women having ADAMTS13 levels less than 5%.[61]
Patients with TTP typically present with non-specific symptoms including easy bruisability, fatigue, nausea, vomiting, and abdominal pain. Neurological manifestations are common (50–80%) and range from mild symptoms, such as lethargy, confusion, and headache, to serious manifestations such as fluctuating focal deficits and seizure.[58] ARF has been reported in 30–80% of women with pregnancy-associated TTP in various case series.[62–64] Although recovery of renal function is typical, persistent renal dysfunction, including need for chronic dialysis or kidney transplantation, is not uncommon.[64] Proteinuria and hematuria are present in the majority of patients with TTP.[65]
The widespread use of plasma exchange for TTP has decreased maternal mortality from 90% to 10%.[66] Antiplatelet agents such as low-dose aspirin or dipyridamole and low-molecular-weight heparin have been used, alone or in combination with glucocorticoids, but none have gained widespread support.[6364] In patients with a prior known history of TTP, serial monitoring of ADAMTS13 levels during pregnancy, with prophylactic plasma exchange and antiplatelet agents if levels are deficient, has been shown to result in good obstetric outcome.[66] For patients who are refractory to these treatments, second-line treatments, including vincristine, rituximab, and splenectomy, have been described during pregnancy, with favorable outcomes.[67] Women who develop TTP/HUS should be aware of the risk of relapse in subsequent pregnancies.[58]
Acute fatty liver of pregnancy
It affects women in the third trimester of pregnancy and is characterized by sudden-onset acute liver failure with coagulopathy. AFLP is estimated to affect between 1/7000 and 1/20,000 pregnancies.[6869] Early diagnosis and immediate delivery are essential for maternal and fetal survival. Maternal and fetal mortality rates were as high as 85% in pregnancies complicated by AFLP in earlier reports.[70] However, recent case series report much lower maternal mortality (0–12.5%).[7172] This improvement in outcome is attributed to earlier diagnosis, intensive obstetric monitoring, and safe emergent delivery of the fetus. Hence, fetal and perinatal mortality rates have also improved, ranging from 6.6% to 15%.[697172]
AFLP characteristically begins in the third trimester with a prodromal phase of nonspecific symptoms including fever, malaise, anorexia, myalgia, nausea, vomiting, and epigastric pain. The severity of liver involvement is variable, ranging from moderate isolated transaminitis to fulminant hepatic failure.[7374] Coagulopathy is a key feature of AFLP, with low fibrinogen level, prolonged prothrombin time, depressed antithrombin III levels, and thrombocytopenia. The other laboratory abnormalities include hyperbilirubinemia, increased transaminases, hypoglycemia, and leukocytosis. AFLP is a diagnosis of exclusion, once viral hepatitis and biliary obstruction have been excluded. The absence of hypertension supports viral hepatitis rather than AFLP.[75] AKI is seen in 20-100% of cases.[72–74] AKI in AFLP is usually nonoliguric.[7374] Proteinuria and peripheral edema are common, as up to 50% have concomitant preeclampsia.[7172]
Early diagnosis, supportive care, and prompt delivery are critical in the management of AFLP. Transfusion of blood products is often needed to correct anemia and coagulopathy. Hepatic encephalopathy is treated with a high-carbohydrate, low-protein diet and oral lactulose. Continuous infusions of 10% dextrose are frequently needed to manage hypoglycemia.[7172] Since most cases resolve spontaneously after delivery, liver transplantation is rarely needed.[7677] Recurrence of AFLP in subsequent pregnancies is rare, with only four cases reported in the literature.[7879]
Differential Diagnosis of Preeclampsia/HELLP Syndrome, HUS/TTP, and AFLP
It is important to distinguish between the causes of AKI in order to make appropriate therapeutic decisions. Typically, AFLP and HELLP syndrome improve after delivery of the fetus, whereas plasma exchange is the first-line treatment for HUS/TTP. Table 4 describes the comparison of laboratory abnormalities in AFLP, HELLP syndrome, and TTP/HUS.

Diagnosis of AFLP from HELLP syndrome
Distinguishing AFLP from HELLP syndrome can be challenging as up to 50% of women with AFLP have concomitant preeclampsia. The two disorders share many pathophysiological and clinical features. In particular, fetal deficiency of long-chain 3-hydroxyacyl-CoA dehydrogenase is a predisposing factor in both conditions, suggesting a common pathophysiological pathway.[80] Even liver biopsy can be non-diagnostic, because findings on liver pathology in HELLP syndrome can include fatty infiltration of the liver, which is the hallmark of AFLP.[8182] The clinical distinction between AFLP and HELLP is arguably academic, since appropriate treatment of both conditions is prompt delivery of the Fetus. Clinical clues to distinguish AFLP vs HELLP syndrome are:
-
AFLP is frequently complicated by coagulopathy and hypoglycemia (key features of AFLP).
-
Thrombocytopenia is moderate to severe in HELLP syndrome, whereas it is mild or absent in AFLP.[72]
Distinguishing TTP from HELLP syndrome
There is substantial clinical and pathological overlap between TTP and preeclampsia/HELLP syndrome, as both have microangiopathic hemolytic anemia and thrombocytopenia [Table 4]. Approximately 20% of women with pregnancy-associated TTP may have concurrent preeclampsia/HELLP syndrome.[58] In addition, maternal mortality in patients with concomitant TTP and preeclampsia/HELLP is very high (44.4%).[58] The following are the clues for the diagnosis of pregnancy-associated TTP:
-
The absence of coagulation abnormalities such as elevated antithrombin, elevated D-dimer, and high fibrinogen levels are suggestive of TTP.
-
Low or undetectable ADAMTS13 level is not helpful for treatment decisions, as levels can be normal in TTP and can be low in HELLP syndrome, without evidence of TTP.[83]
Renal Cortical Necrosis in Pregnancy
Renal cortical necrosis (RCN) is a rare entity that accounts for only 2% of all cases of ARF in developed nations.[84] RCN is secondary to ischemic necrosis of the renal cortex, and lesions are usually caused by significantly diminished renal arterial perfusion resulting from vascular spasm, microvascular injury or intravascular coagulation. Obstetric complications are the commonest (50–70%) causes of RCN;[8586] while non-obstetric causes account for 20–30% of all cases of cortical necrosis. Abruptio placentae, septic abortion, eclamptic toxemia, postpartum hemorrhage, and puerperal sepsis are the pregnancy-related situations responsible for RCN.[558487] Obstetric causes were responsible for RCN in 56% and 61% cases in our previous two studies.[4288] However, incidence of cortical necrosis in PR-ARF has decreased (P<0.001) from 17% in 1982–1991 to 2.4% in the 2000s[39] [Figure 1]. In our recent study, the overall incidence of RCN in obstetric ARF was 15.2%, and it had decreased significantly from 4.7% in 1984–1994 to 0.5% in 1999–2005 of total ARF cases.[3942] Obstetric causes accounted for 9% of cases of cortical necrosis in patients with PR-ARF in Pakistan.[8990] The gold standard for establishing the diagnosis of cortical necrosis is renal histology; which showed ischemic necrosis of all elements of renal parenchyma of the cortical region [Figure 2]. However, contrast-enhanced computed tomography (CT) scan has emerged as a good non-invasive modality for early diagnosis of RCN in recent years. The characteristic finding is the presence of hypo-attenuated subcapsular rim of renal cortex following contrast injection [Figure 3]. In addition, a non-contrast CT can is more sensitive in picking up the cortical calcification.
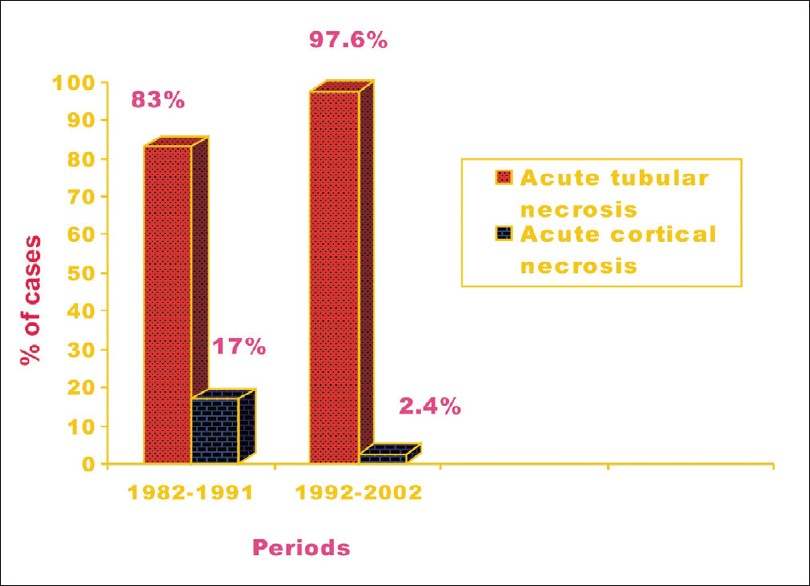
- Renal cortical necrosis decreased to 2.4% in 1992-2002 from 17% in 1982-1991 in obstetric acute renal failure
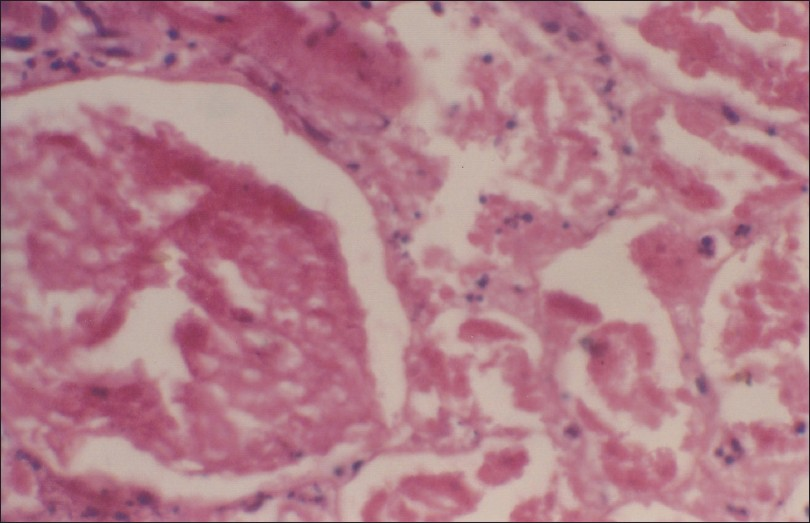
- Renal histology showing ischemic necrosis of all elements of renal parenchyma in the cortex; typical feature of cortical necrosis (H and E, 400×)

- Contrast enhanced CT scan showing uniform hypoattenuated subcapsular rim of cortex, the characteristic feature of renal cortical necrosis
Septic abortion continues to be an important cause of RCN, possibly due to endotoxin-mediated endothelial damage leading to vascular thrombosis and subsequent renal ischemia. Abruptio placentae is the most common obstetric cause of RCN, accounting for 50–60% of cases in developed countries.[9091] However, RCN is very rare following septic abortion in developed countries but a common obstetric cause of RCN in our country.[47489293] The reasons for this discrepancy is the fact that abortions are commonly conducted by untrained person in unhygienic setting, accounting for a high incidence of septic abortions and sepsis-related complications.[4787]
Conclusion
Incidence of obstetric ARF has declined and septic abortion-related ARF has markedly decreased in our country. Majority of PR-ARF were observed in the late pregnancy or postpartum period. Preeclampsia and puerperal sepsis are the major cause of ARF in the postpartum period. Septic abortion was the main cause of ARF in early pregnancy in past; however, incidence of postabortal ARF has decreased. ARF in pregnancy is largely a preventable problem. The timely and aggressive management of obstetrical complications will certainly reduce the incidence of AKI in pregnancy. The differential diagnosis of thrombotic microangiopathy with AKI in pregnancy is challenging.
UTI is a serious infection during pregnancy, and if untreated UTI is associated with several adverse maternal and fetal outcomes.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Hypertension and Kidney Disease in Pregnancy. In: Brenner BM, Rector B, eds. The Kidney (8th ed). Philadelphia: Saunders Elsevier; 2008. p. :1567-95.
- [Google Scholar]
- World Health Organization. In: World Health Report: make every mother and child count. Geneva: WHO; 2005.
- [Google Scholar]
- Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24-8.
- [Google Scholar]
- The evolution of the treatment of eclampsia. J Obstet Gynaecol Br Commonw. 1961;68:417-26.
- [Google Scholar]
- Hypertension in pregnancy: hospital based study. J Assoc Physicians India. 2006;54:273-8.
- [Google Scholar]
- Spectrum of kidney diseases in patients with preeclampsia-eclampsia. J Assoc Physicians India. 2010;58:543-6.
- [Google Scholar]
- Epidemiology of preeclampsia and eclampsia in the United States, 1979-1986. Am J Obstet Gynecol. 1990;163:460-5.
- [Google Scholar]
- Diagnosis, prevention and management of eclampsia. Obstet Gynecol. 2005;105:402-10.
- [Google Scholar]
- Late postpartum eclampsia: A preventable disease? Am J Obstet Gynecol. 2002;186:1174-7.
- [Google Scholar]
- ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynecol Obstet. 2002;77:67-75.
- [Google Scholar]
- Proteinuria in preeclampsia: How much matter? Br J Obstet Gynaecol. 2005;112:280-3.
- [Google Scholar]
- Preeclamptic nephropathy- an endothelial lesion - A morphological study with a review of the literature. Eur J Obstet Gynecol Reprod Biol. 1988;77:11-27.
- [Google Scholar]
- Clinical evolution of hypertension and proteinuria in patients who developed preeclampsia. Int J Gynaecol Obstet. 2005;5:1.
- [Google Scholar]
- Glomerular ultrafiltration in normal and preeclamptic pregnancy. J Am Soc Nephrol. 2003;14:648-52.
- [Google Scholar]
- Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981-91.
- [Google Scholar]
- Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-306.
- [Google Scholar]
- The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol. 1999;180:1373-84.
- [Google Scholar]
- Acute renal failure in pregnancies complicated by hemolysis, elevated liver enzymes, and low platelets. Am J Obstet Gynecol. 1993;168:1682-7. discussion 1687-90
- [Google Scholar]
- Acute renal failure complicating severe preeclampsia requiring admission to an obstetric intensive care unit. Am J Obstet Gynecol. 2002;186:253-6.
- [Google Scholar]
- Maternal and fetal outcomes in HELLP syndrome complicated with acute renal failure. Ren Fail. 2004;26:557-62.
- [Google Scholar]
- Risk factors for adverse maternal outcomes among women with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:444-8.
- [Google Scholar]
- Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome) Am J Obstet Gynecol. 1993;169:1000-6.
- [Google Scholar]
- Cardiovascular sequelae of preeclampsia/eclampsia: a systemic review and meta-analyses. Am Heart J. 2008;156:918-30.
- [Google Scholar]
- Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800-9.
- [Google Scholar]
- Epidemiology of preeclampsia and eclampsia in the United States, 1979-86. Am J Obstet Gynecol. 1990;163:460-5.
- [Google Scholar]
- Management of hypertension during pregnancy. In: Largh GN, Brenner BM, eds. In: Hypertension: Pathophysiology Diagnosis and Management (1st ed). New York: Raven; 1990. p. :1809-27.
- [Google Scholar]
- ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. ACOG Committee on Practice Bulletins. Obstet Gynecol. 2001;98(suppl):117-85.
- [Google Scholar]
- Urinary tract infection complicating pregnancy. Infect Dis Clin North Am. 1997;11:13-26.
- [Google Scholar]
- Causes of the excessive rates of perinatal mortality and prematurity in pregnancies complicated by maternal urinary-tract infections. N Engl J Med. 1979;300:819-23.
- [Google Scholar]
- Antibiotic for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001;2:CD000490.
- [Google Scholar]
- Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 2000;2:CD000491.
- [Google Scholar]
- Urinary tract infection during pregnancy and fetal outcome. Indian J Med Microbiol. 1996;14:158-60.
- [Google Scholar]
- Epidemiologic trend changes in acute renal failure- a tertiary center experience from South India. Ren Fail. 2006;28:405-10.
- [Google Scholar]
- Acute renal failure in pregnancy in a developing country: twenty years experience. Ren Fail. 2006;28:309-13.
- [Google Scholar]
- Changes in frequency and etiology of acute renal failure in pregnancy (1980-1997) Ren Fail. 1998;20:513-7.
- [Google Scholar]
- Is pregnancy-related acute renal failure a disappearing entity? Ren Fail. 1996;18:575-84.
- [Google Scholar]
- Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries. a single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant. 2007;22:1213-7.
- [Google Scholar]
- Changing trends in acute renal failure in third-world countries--Chandigarh Study. Q J Med. 1989;73:1117-23.
- [Google Scholar]
- Severe sepsis and septic shock in pregnancy and puerperium: An 11-years review in a maternity intensive care unit. Crit Care. 2007;11(Suppl 4):39.
- [Google Scholar]
- Acute kidney injury in late pregnancy in developing countries. Ren Fail. 2010;32:309-13.
- [Google Scholar]
- Pregnancy-related acute renal failure: A single-centre experience. Indian J Nephrol. 2008;18:17-21.
- [Google Scholar]
- The natural history of HELLP syndrome: patterns of disease progression and regression. Am J Obstet Gynecol. 1991;164:1500-9. discussion 1509-13
- [Google Scholar]
- Postpartum renal failure: a complex case with probable coexistence of hemolysis, elevated liver enzymes, low platelet count, and hemolytic uremic syndrome. Obstet Gynecol. 1998;92:698-700.
- [Google Scholar]
- Pathogenesis of acute renal failure associated with the HELLP syndrome: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003;108:99-102.
- [Google Scholar]
- Acute Renal Failure and HELLP Syndrome: A single centre's experience. Saudi J Kidney Dis Transpl. 1998;9:290-3.
- [Google Scholar]
- Spectrum of renal cortical necrosis in acute renal failure in eastern India. Postgrad Med J. 1995;71:208-10.
- [Google Scholar]
- Outcome of pregnancies with HELLP syndrome complicated by acute renal failure (1989-1999) Ren Fail. 2000;22:319-27.
- [Google Scholar]
- Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354:1927-35.
- [Google Scholar]
- The association of pregnancy with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2003;10:339-44.
- [Google Scholar]
- Thrombotic thrombocytopenic purpura in 166 pregnancies: 1955-2006. Am J Obstet Gynecol. 2008;199:98-104.
- [Google Scholar]
- Pregnancy outcomes after recovery from thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Transfusion. 2004;44:1149-58.
- [Google Scholar]
- ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60-8.
- [Google Scholar]
- Thrombotic thrombocytopenic purpura and pregnancy: a review of ten cases. Vox Sang. 2004;87:287-90.
- [Google Scholar]
- Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome in pregnancy: review of 11 cases. Am J Obstet Gynecol. 1996;175:950-6.
- [Google Scholar]
- The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancy. Obstet Gynecol. 1998;91:662-8.
- [Google Scholar]
- Renal involvement in patients with thrombotic thrombocytopenic purpura. Am J Nephrol. 1986;6:117-31.
- [Google Scholar]
- The thrombotic thrombocytopenic purpura and hemolytic uremic syndrome: evaluation, management, and long-term outcomes experience of the Oklahoma TTP-HUS registry, 1989-2007. Kidney Int Suppl. 2009;112:S52-4.
- [Google Scholar]
- Acute kidney injury in pregnancy: the thrombotic microangiopathies. J Nephrol. 2011;24:554-63.
- [Google Scholar]
- Acute fatty liver of pregnancy: a clinical study of 12 episodes in 11 patients. Gut. 1994;35:101-6.
- [Google Scholar]
- UK Obstetric Surveillance System.A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57:951-6.
- [Google Scholar]
- Maternal and perinatal outcome in severe pregnancy-related liver disease. Hepatology. 1997;26:1258-62.
- [Google Scholar]
- Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192:1416-9.
- [Google Scholar]
- Reversible peripartum liver failure: a new perspective on the diagnosis, treatment, and cause of acute fatty liver of pregnancy, based on 28 consecutive cases. Am J Obstet Gynecol. 1999;181:389-95.
- [Google Scholar]
- Acute fatty liver of pregnancy.An experience in the diagnosis and management of fourteen cases. Am J Obstet Gynecol. 1994;171:1342-7.
- [Google Scholar]
- Pregnancy-associated acute liver disease and acute viral hepatitis: differentiation, course and outcome. J Hepatol. 2008;49:930-5.
- [Google Scholar]
- Renal morphological changes in idiopathic acute fatty liver of pregnancy. Histopathology. 1984;8:567-81.
- [Google Scholar]
- Acute fatty liver of pregnancy associated with preeclampsia: management of hepatic failure with postpartum liver transplantation. Am J Perinatol. 1991;8:278-9.
- [Google Scholar]
- Recurrence of acute fatty liver of pregnancy. Br J Obstet Gynaecol. 1994;101:453-4.
- [Google Scholar]
- A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med. 1999;340:1723-31.
- [Google Scholar]
- Study of the liver changes occurring in preeclampsia and their possible pathogenetic connection with acute fatty liver of pregnancy. Am J Gastroenterol. 1996;91:292-4.
- [Google Scholar]
- Preeclampsia: a microvesicular fat disease of the liver? Am J Obstet Gynecol. 1988;159:1043-7.
- [Google Scholar]
- Mild to moderate reduction of a von Willebrand factor cleaving protease (ADAMTS-13) in pregnant women with HELLP microangiopathic syndrome. Haematologica. 2003;88:1029-34.
- [Google Scholar]
- Acute cortical necrosis: Case report and review of the literature. Am J Med. 1974;56:110-8.
- [Google Scholar]
- Diagnostic procedures and long-term prognosis in bilateral cortical necrosis. Kidney Int. 1973;4:390-400.
- [Google Scholar]
- Renal cortical necrosis in pregnancy-related acute renal failure. J Indian Med Assoc. 1996;94:227-9.
- [Google Scholar]
- Acute renal failure of obstetrical origin during 1994 at one center. Ren Fail. 1996;18:681-3.
- [Google Scholar]
- Acute renal failure of obstetric origin: An analysis of 70 patients. In: Lancet. Vol 2. 1965. p. :351-4.
- [Google Scholar]
- Spectrum of acute cortical necrosis in Indian patients. Am J Med Sci. 1983;286:10-20.
- [Google Scholar]







