The Prevalence and Outcomes of BK Polyoma Virus Nephropathy in Living Donor Kidney Transplant Recipients
*Abhyuday Rana & Shyam B Bansal contributed equally and shall be the first co-authors.
Corresponding author: Shyam Bihari Bansal, Department of Nephrology and Kidney Transplantation, Medanta Institute of Kidney and Urology, Medanta—The Medicity, Gurgaon, India. E-mail: drshyambansal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rana A, Bansal SB, Kotton CN, Mahapatra AK, Rana A, Sethi SK, et al. The Prevalence and Outcomes of BK Polyoma Virus Nephropathy in Living Donor Kidney Transplant Recipients. Indian J Nephrol. doi: 10.25259/ijn_87_23
Abstract
Background
BK polyomavirus nephropathy (BKPyVN) remains an important cause of allograft dysfunction and loss. There is little data about prevalence and outcome of BKPyVN infection from India in living donor kidney transplant recipients.
Materials and Methods
This is a retrospective analysis of all biopsy-proven BKPyVN among kidney transplant recipients at our center from January 2010 to January 2022. We compared them to age, sex, and type of immunosuppression received matched (1:2) non-BKPyVN-infected recipients transplanted during the same period.
Results
During the study period, 2465 patients underwent kidney transplants at our center, of which 26 (1.05%) developed biopsy-proven BKPyVN. Four recipients (16%) lost their graft over a median period of 65 (IQR, 57–83) months from the time of diagnosis. The mean serum creatinine at the recent follow-up was higher in the BKPyVN arm as compared to controls (2.05 ± 1.39 vs 1.35 ± 0.46, p = 0.001.) Both BKPyVN and control arms had similar death-censored graft survival (82% vs 94%, p = 0.09) and patient survival (88% vs 96%, p = 0.184).
Conclusion
BKPyVN was uncommon in our kidney transplant recipients. Most patients were able to maintain their kidney function for many years, albeit at a somewhat reduced level compared with the controls, and about a fifth of our patients lost their graft.
Keywords
BK virus nephropathy
Kidney transplant
Post-transplant viral infection
India
Living donor
Introduction
Kidney transplant is the best modality of kidney replacement therapy for most patients with kidney failure, although this is accompanied by an augmented risk of opportunistic infections.1,2 BK polyomavirus (BKPyV) is one such opportunistic viral infection causing an increased risk of graft dysfunction and graft loss in kidney transplant recipients.1,2 The global incidence of BKPyV viruria post kidney transplant has been reported to be between 30% and 60%, and BKPyV viremia has been reported to be around 20–30%; however, biopsy-proven BK polyomavirus nephropathy (BKPyVN) is seen in 1% 10% in various studies after kidney transplantation.3–7 The incidence varies depending on the choice of induction, maintenance immunosuppression, screening modality and other factors.3–7
Kidney allograft biopsy remains the gold standard for diagnosing “definite” BKPyVN.7,8 BKPyVN is defined as probable when the blood BKPyV quantitative polymerase chain reaction (PCR) value is >103 log on two separate occasions three weeks apart and “presumptive” is defined as persistently high BKPyV viral load in plasma >10,000 copies/mL for four weeks in one of the two measurements.7 The graft loss due to BKPyVN was reported to be 50–100% in initial studies; however, recent studies have reported graft loss between 10% and 50% with aggressive screening and management for BKPyV infection.3,5,6,9,10
There are some data about BKPyV infection from India, specifically in living donor kidney transplant recipients; however, they don’t have any outcome data.11–14 There is no consensus about optimal immunosuppression management after control of BKPy viremia and nephropathy. We analyzed our center’s experience with biopsy-proven BKPyVN and compared them to age, sex, and type of immunosuppression (induction and maintenance) received matched (propensity matched 1:2) non-BKPyV-infected recipients transplanted during the same period.
Materials and Methods
This is a retrospective analysis of prospectively collected data of living donor kidney transplant recipients from Medanta Medicity Hospital Gurugram, which is a large multi super speciality tertiary care centre in India. All patients transplanted at our center from January 2010 to January 2022 were included in the study. The medical records were retrieved and the demographic findings along with clinical and histopathology data were reviewed. Data collection was stopped for analysis on April 1, 2022. No routine screening/surveillance of serum or urine BKPyV quantitative PCR is done at our center due to financial considerations. Graft biopsy for indication, usually for graft dysfunction, defined as a 25% or more increase in serum creatinine. Histological diagnosis was made by the prevailing Banff criteria and later during the preparation of the manuscript; all biopsies were reviewed by our pathologist and reclassified to classes as per current the Banff criteria.8
BKPyVN quantitative PCR was done at the time of confirmation of diagnosis and then at follow-up, as per the treating nephrologist’s discretion.
Patients with biopsy-proven BKPyVN were compared controls matched for age, sex, type of immunosuppression and transplant vintage. Patients with BKPyV viremia (n = 21) without biopsy-proven nephropathy were excluded.
All patients were initially on Tacrolimus (TAC) + mycophenolate mofetil (MMF) + Steroid (except for few patients on early steroid minimization protocol). After diagnosis of BKPyVN, MMF was stopped and reintroduced (on the treating physician discretion) only 1-month after disappearance of viremia. In case of reappearance of BK viremia, MMF was permanently stopped. Patients were followed up till April 1, 2022. The outcomes evaluated were kidney function, biopsy-proven acute and chronic rejection, graft failure, and patient survival.
As it was a retrospective observational study, it was exempted from the ethics committee clearance from our institute. During the whole process of research, the confidentiality and privacy of participants were assured. The study abided by the rules of the declaration of Helsinki, and the declaration of Istanbul.
Statsistical analysis
Qualitative variables are expressed as absolute numbers and percentages and quantitative variables are expressed as mean ± SD or as median (interquartile range [IQR]). Appropriate tests for statistical significance were used for comparisons between various groups—the Chi-square test or Fisher’s exact test for qualitative data, the Independent samples t-test for continuous variables and the Mann-Whitney U test for nonparametric data. For categorical variables, the Chi-square test or Fisher’s exact test was done. A P value of <0.05 was considered to be statistically significant. Statistical analysis was done with SPSS, version 20.0.
Results
A total of 2465 were transplanted during the study period of which 26 (1.05%) were found to have biopsy-proven BKPyVN. The mean age of the recipients was 38.1 ± 15 years and the male-to-female ratio was 5.5:1. All patients had undergone living-related donor kidney transplants, five (19%) were preemptive transplants, two (8%) were ABO incompatible, and one (4%) was Human Leucocyte antigen (HLA) incompatible who underwent desensitization before kidney transplant [Table 1].
| Baseline demographics | |||
|---|---|---|---|
| BKPyV patients (n = 26) | Matched controls (n = 52) | P value | |
| Age (Mean ± SD) years | 38.1 ± 15 | 38.5 ± 13 | 0.9 |
| Male-to-female ratio | 22:4 | 45:7 | 0.7 |
| Dialysis vintage months (Median ± IQR) | 2 (1–4) | 3 (1–5) | 0.8 |
| Donor age (Mean ± SD) years | 47.5 ± 11.2 | 46.02 ± 9.3 | 0.53 |
| Type of transplant | |||
| Preemptive | 5 (19%) | 11 (21%) | 0.83 |
| Retransplant | 0 | 0 | - |
| LDRT | 26 (100%) | 52 (100%) | 1 |
| ABO i | 2 (8%) | 4 (8%) | 1 |
| HLA i | 1 (4%) | 1 (2%) | 0.6 |
| Induction agent | |||
| None | 5 (19%) | 11 (21%) | 0.83 |
| Basliximab | 13 (50%) | 27 (52%) | 0.86 |
| ATG (Thymoglobulin) | 6 (23%) | 12 (23%) | 1 |
| ATG F (Grafalon) | 2 (8%) | 2 (4%) | 0.46 |
| Maintenance regimen | |||
| TAC+MMF+ S | 24 (92%) | 49 (94%) | 0.74 |
| Steroid free | 2 (8%) | 3 (6%) | 0.7 |
| Mean HLA mismatch | 4.07 ± 1.38 | 3.02 ± 1.43 | |
LDRT: Living donor (related) renal transplant, ABO i: ABO incompatible, HLA: Human leucocyte antigen, HLA i: HLA incompatible, ATG: Anti-thymocyte globulin, TAC: Tacrolimus, MMF: Mycophenolate Mofetil, S: Steroid, BKPyV: BK polyomavirus, ATG F: antithymocyte globulin fresenius, IQR: interquartile range
Among the 26 recipients, 13 received basiliximab, six received rabbit anti thymocyte globulin-fresenius (Thymoglobulin®: Sanofi, 3mg/kg in two divided doses), and two received ATG-F (Grafalon-Zydus) at 6 mg/kg (two divided doses), while five did not receive any induction immunosuppression. Twenty-four patients (92%) were on a triple immunosuppressant maintenance regime comprising tacrolimus (TAC), MMF, and steroids while two (8%) were on early steroid-withdrawal regimen (TAC + MMF). There was no difference in baseline characteristics between the patient and control groups in most parameters, including induction and maintenance immunosuppression showing that the groups were well matched The only significant difference was higher number of HLA mismatches in the BKPyVN arm (p = 0.28) [Table 1].
The median time of development of BKPyVN was 23 (18–28) months and the median BKPy viremia at diagnosis (Median, Q1–Q3) was 70,710 (36,860–175,300) DNA copies/ml. Besides stopping MMF, we also reduced TAC doses in these patients. The median tacrolimus trough level at the time of diagnosis was 7.05 (6.45–7.75) ng/ml, which came down to 5 (4.3–5.6) ng/ml at the time of disappearance of serum BKV DNA. From our cohort of 26 patients, ten patients had class 1 BKPyVN, 11 had class 2 BKPyVN, and five had class 3 BKPyVN according to Banff criteria [Figure 1]. Of the five patients with class 3 BKV nephropathy, four lost their grafts [Table 2].
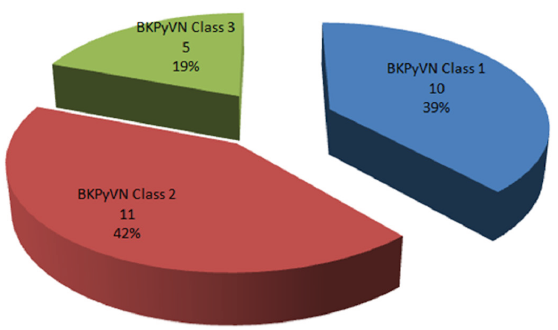
- BK Polyoma virus nephropathy (BKPyVN) class according to biopsy.
| BKV Nephropathy management | |
|---|---|
| Median duration time for diagnosis from date of transplant (Median, Q1–Q3) months | 23 (18–28) months |
| Median BK polyoma viremia at diagnosis (Median, Q1–Q3) DNA copies/ml | 70,710 (36,860–175,300) DNA Copies/ml |
| Discontinuation of MMF (n) | 26 (100%) |
| Median time for clearance of BKV DNA copies (Median, Q1–Q3) months | 6.5 (4.25–8.75) months |
| Median Tacrolimus trough level at time of diagnosis (Median, Q1–Q3) in ng/ml | 7.05 (6.45–7.75) mg/ml |
| Undetectable serum BKPyV DNA PCR at last follow-up | 16 (70%) of the 23 surviving patients |
| Median tacrolimus trough level at the time of disappearance of serum BKV DNA copies in (Median, Q1–Q3) ng/ml | 5 (4.3–5.6) ng/ml |
| Mean time of development of acute rejection after BKPyVN (months) | 6.2 ± 2.8 |
| Graft loss (n) | 4 (15%) |
| Retransplant | None |
BKPyV: BK Plyoma virus, BKV DNA PCR: BK virus DNA PCR, MMF: mycophenolate mofetil
Excluding those who lost graft function and/or who died, the mean serum creatinine at the last follow-up was higher in the BKPyVN group as compared to the control group (2.05 ± 1.39 vs 1.35 ± 0.46, p = 0.0015) with a lower estimated GFR (42 ± 19 vs 69 ± 20, p = 0.0001). No episodes of delayed graft function (DGF) or biopsy proven rejection prediagnosis of BKPyVN occurred and none of the patients had lymphopenia before the diagnosis of BKPyVN.
Patients with BKPyVN had a higher incidence of biopsy-proven acute rejection (30% vs 10%, p = 0.02) within 1 year of BKPyVN diagnosis; however, there was no difference in chronic rejection rates till the last follow-up [Table 3]. The mean time of development of AR was 6.2 ± 2.8 months after BKPyVN. BKV monitoring was done in patients with AR, and two of them developed viremia after treatment of AR; subsequently, immunosuppression was reduced in these patients. The incidence of infections and other complications were similar in both groups [Table 3].
| Follow-up period median-65 (IQR, 57–83) months | |||
|---|---|---|---|
| BKPyV patients (n = 26) | Matched controls (n = 52) | p value | |
| Patient survival (%) | 23 (88%) | 50 (96%) | 0.184 |
| Death censored graft survival (%) | 22 (82%) | 49 (94%) | 0.09 |
| S creatinine on last follow-up (Mean ± SD), mg/dl | 2.05 ± 1.39 | 1.35 ± 0.46 | 0.0015* |
| Estimated glomerular filtration rate at last follow-up (Mean ± SD), ml/min/1.73m2 | 42 ± 19 | 69 ± 20 | 0.0001 |
| Biopsy-proven rejection (n, %) BKPyVN | 10 (38%) | 8 (15%) | 0.02* |
| Acute rejection (n, %) | 8 (30%) | 5 (10%) | 0.02* |
| ACR 1a | 5 | 4 | 0.6 |
| ACR 1b | 2 | 1 | |
| ACR 2a | 1 | 0 | |
| ACR 2b | 0 | 0 | |
| ACR 3 | 0 | 0 | |
| ABMR | 0 | 0 | |
| Chronic rejection (n, %) (Chronic ABMR) | 2 (8%) | 3 (5%) | |
| Infections (n, %) | 4 (15%) | 7 (13%) | 0.8 |
| UTI | 2 | 4 | |
| LRTI | 1 | 2 | |
| Tuberculosis | 0 | 1 | |
| Others | 1 | 0 | |
| CMV infection (n, %) | 0 | 1 (2%) | 0.47 |
| Post-transplant malignancy (n, %) | 0 | 0 | - |
| NODAT (n, %) | 3 (12%) | 5 (10%) | 0.7 |
UTI: Urinary tract infection, LRTI: Lower respiratory tract infection, CMV: Cytomegalovirus, ABMR: Antibody mediated rejection, IQR: interquartile range, NODAT: New onset diabetes after transplant, *: statistically significant value, ACR: Acute cellular rejection.
Four patients (16%) lost their graft over a median period of 65 (IQR, 57–83) months from the time of diagnosis and three (12%) patients died with a functioning graft due to either sepsis (n = 1, 4%) or cardiac events (n = 2, 8%) in the BKPyVN group. The graft survival was not significantly different, but there was a trend for more graft loss in BKPyVN group as compared to control (18% vs 6%, p = 0.009) [Figure 2].
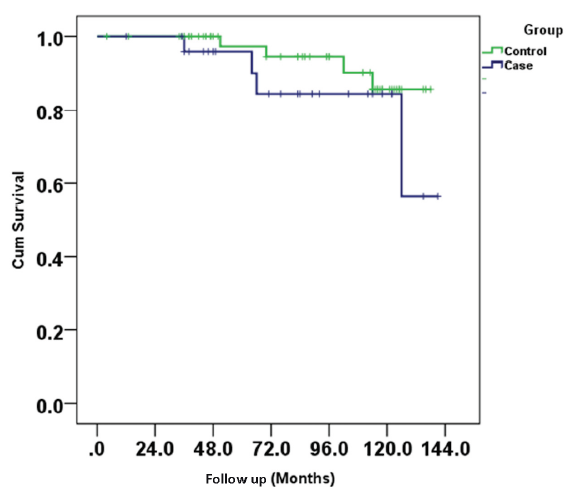
- Kaplan meier curve showing patient survival between two groups.
Both the BKPyVN and control arms had similar patient survival (88% vs 96%, p = 0.184) [Figure 3]. Serum BKPyV DNA PCR was undetectable in 16 out of 23 surviving patients. None of the patients who lost their grafts due to BKV nephropathy underwent retransplantation.
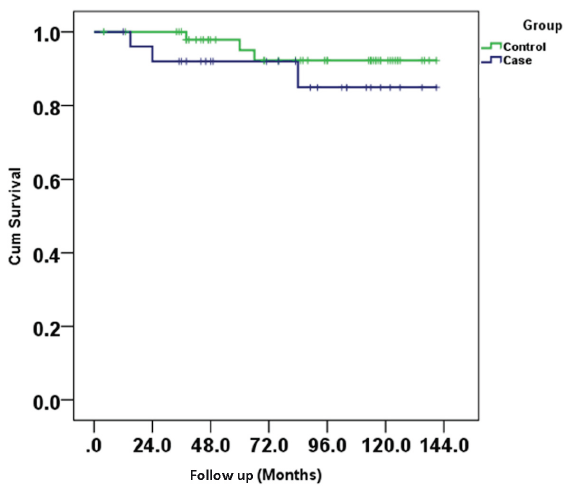
- Kaplan meier curve showing graft/kidney survival between two groups.
Discussion
BKPyV is a common and challenging opportunistic infection causing allograft dysfunction and loss.3–7 Several factors, including allograft, viral, and host, influence the reactivation of BKV, and allograft damage.10,12
The incidence of biopsy-proven BKPyVN was reported from the registry data from the west ranges between 1% and 10%.3–6,9–10 In the absence of a common registry and a lack of uniform screening protocol in India, the reported incidences of BKPyVNfrom retrospective single-center cohorts or case series range from 1.49% to 9.3%.11–14 In our cohort, the incidence of biopsy-proven BKPyVN was 1.05%, which is lower than the two previously reported series from India but almost similar to the study by Gupta et al., which is a more recent study. The initial two studies were conducted before 2010 and there was a lot of reported acute rejection, and it is possible some of these patients could have only one pathology as histopathological features of acute rejection and BKPyVN can be similar.8 The new guidelines by the American Society of Transplantation, infectious diseases consider concomitant acute rejection only when features like C4d positivity, glomerulitis, and endotheliatis are present.7,8 We did not review all biopsies in our patients, due to which some patients without graft dysfunction might have been missed; however, we biopsied all our patients with graft dysfunction. We excluded patients with only viremia without graft dysfunction and did not do biopsies on them.
BKPyVN occurs most commonly in the first two months to two years posttransplantation or following treatment of acute rejection.3–7 In our cohort, the median time duration for diagnosis from the date of the transplant was 23 (18–28) months, similar to the other studies.3–7,11–17
Th postulated risk factors for the development of BKPyVN, include the use of thymoglobulin, higher steroid use, use of tacrolimus as maintenance immunosuppression and DGF, Some donor and recipient-related risk factors include male sex, younger or elderly recipient, deceased donor, BKV positivity in donor, and increasing HLA mismatch.3–4,10,16–18 The most common induction agent in our patients was interleukin 2 receptor antibody basiliximab. All our patients were living-related donor transplants with good immunological matching and were kept on our standard triple maintenance regimes consisting of TAC, MMF, and low-dose steroids; however, we did not find any difference in BKPyVN versus control group in these parameters. We could only find higher number of HLA mismatches in BKPyVN arm as compared to controls.
Kidney biopsy remains the gold standard for diagnosing BKPyVN; however, histological confirmation can be difficult due to the focal nature of the infection resulting in false negative results in 10–30% of biopsies.8,19 We reclassified our patients according to new Banff criteria and found that out of the five patients with class 3 BKPyVN, four lost their graft, while none of patients in class 1 or 2 lost their grafts, highlighting that allograft survival in patients with BKPyVN is worse in those with advanced diseases, thus warranting early screening and diagnosis.7,20
A reduction in the intensity of immunosuppression is the mainstay for the treatment of BKPyV viremia and BKPyVN.5,7,21–25 Multiple protocols have been described for reduction in immunosuppression. Some centers advocate reduction in CNI followed by antiproliferative agents, others advocate the reverse.5,23,25 Our center’s protocol is to reduce the antiproliferative agents (MMF/azathioprine) in case of viremia with no BKPyVN. In case of failure of decline in the viral load or a biopsy-proven BKV nephropathy, a combination of the cessation of an antiproliferative agent with reduction of TAC dose to maintain a trough level of 4–6 ng/mL is done.
Careful monitoring of graft function is required during the follow-up period, as the reduction of immunosuppression can lead to increased rejections.25,26 In our cohort, 8/26 (30%) patients developed biopsy-proven acute rejection while two (8%) developed chronic rejection after immunosuppression reduction which was significantly higher than the control group.
We compared our BKPyVN cohort with age, sex, and type of immunosuppression matched KTR transplanted during the same time for comparison of patient and graft outcomes. In our cohort, during the median 65 (IQR, 57–83) months from the time of diagnosis, four patients (16%) lost their grafts, making the death-censored graft survival to be 82%, which was statistically not significant. Still, it was higher than non-BKPyVN. The patient survival was similar between the groups. The graft function was worse in BKPyVN group as compared to controls. We did not use any other treatment in our patients as it has not been found to be effective and not recommended.7,20
Conclusion
Biopsy-proven BKPyVN is not so common in our kidney transplant recipients. With early detection and prompt management, the graft loss is not so common and about a fifth of our patients with BKPyVN lost their kidney graft. Most patients were able to maintain their kidney function for many years, albeit at a somewhat reduced function compared with the controls. Acute rejection was high as compared to control after the reduction of immunosuppression.. Larger prospective series with active screening which can assess the risk factors and compare various treatment modalities and longer follow-up are needed to guide transplant clinicians in determining a cost-effective screening protocol for early detection and management of this preventable cause of allograft loss.
Conflicts of interest
There are no conflicts of interest.
Authors contributions
SB and AR participated in research design, AR and SB participated in the writing of the paper, SB, CNK, DY, DB, PJ, VK, AG, and MJ participated in the review of manuscript, AR and SB participated in data collection and analysis.
References
- Infection in organ transplantation. Am J Transplant. 2017;17:856-79.
- [CrossRef] [PubMed] [Google Scholar]
- Viral infection in the renal transplant recipient. J Am Soc Nephrol.. 2005;16:1758-74.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488-96.
- [CrossRef] [PubMed] [Google Scholar]
- An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019-26.
- [CrossRef] [PubMed] [Google Scholar]
- BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol. 2007;2:S36-S46.
- [CrossRef] [PubMed] [Google Scholar]
- The decade of polyomavirus BK-associated nephropathy: State of affairs. Transplantation. 2009;87:621-30.
- [CrossRef] [PubMed] [Google Scholar]
- BK polyomavirus in solid organ transplantation—guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13528.
- [CrossRef] [PubMed] [Google Scholar]
- The Banff working group classification of definitive polyomavirus nephropathy: Morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29:680-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582-94.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J Clin Cases. 2019;7:270.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The high incidence of BK polyoma virus infection among renal transplant recipients in India. Transplantation. 2004;77:429-31.
- [CrossRef] [PubMed] [Google Scholar]
- BK virus: Discovery, epidemiology, and biology. Graft-Georgetown. 2002;5:S19-27.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Polyomavirus nephropathy and cytomegalovirus nephritis in renal allograft recipients. Indian J Pathol Microbiol. 2010;53:672.
- [CrossRef] [PubMed] [Google Scholar]
- BK Virus nephropathy in living donor renal allograft recipients: An observational study from a large transplant center in India. Saudi J Kidney Dis Transpl. 2018;29:1366-70.
- [CrossRef] [PubMed] [Google Scholar]
- Histological patterns of polyomavirus nephropathy: Correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082-92.
- [CrossRef] [PubMed] [Google Scholar]
- Polyomavirus BK infection in pediatric kidney-allograft recipients: A single-center analysis of incidence, risk factors, and novel therapeutic approaches. Transplantation. 2003;75:1266-70.
- [CrossRef] [PubMed] [Google Scholar]
- Pretransplantation donor-recipient pair seroreactivity against BK polyomavirus predicts viremia and nephropathy after kidney transplantation. Am J Transplant. 2017;17:161-72.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for BK polyoma virus treatment and association of treatment with kidney transplant failure: Insights from a paired kidney analysis. Transplantation. 2016;100:854-61.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in BK virus complications in organ transplantation and beyond. Kidney Med. 2020;2:771-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1-S155.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of polyomavirus infection in kidney transplant recipients: A systematic review. Transplantation. 2010;89:1057-70.
- [CrossRef] [PubMed] [Google Scholar]
- Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615-23.
- [CrossRef] [PubMed] [Google Scholar]
- Does reduction in immunosuppression in viremic patients prevent BK virus nephropathy in de novo renal transplant recipients? A prospective study. Transplantation. 2008;85:1099-104.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment for BK virus: Incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22:626-34.
- [CrossRef] [PubMed] [Google Scholar]
- Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: Long-term outcomes. Nephrol Dial Transplant. 2019;34:1240-50.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors of acute rejection in patients with BK nephropathy after reduction of immunosuppression. Ann Transplant. 2018;23:704-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








