Translate this page into:
The Relationship between Metabolic Acidosis and Nutritional Parameters in Patients on Hemodialysis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The progressive loss of kidney function is accompanied by metabolic acidosis. The relationship between metabolic acidosis, nutritional status, and oral bicarbonate supplementation has not been assessed in the Indian chronic kidney disease (CKD) population who are on maintenance hemodialysis (MHD). This is a single-center prospective study conducted in the Western part of India. Thirty-five patients, who were receiving MHD were assessed for metabolic acidosis along with various nutritional parameters at the baseline and at the follow-up after 3 months, postcorrection of acidosis with oral sodium bicarbonate supplements. The relationship between the correction of metabolic acidosis with oral bicarbonate supplements and changes in dietary and various nutritional parameters were evaluated. Metabolic acidosis at the baseline evaluation was found in 62.86% cases of the cohort with a mean serum bicarbonate value of 20.18 ± 4.93 mmol/L. The correction of acidosis with increment in the mean dosage of oral sodium bicarbonate supplements from 0.69 ± 0.410 mmol/kg/day at baseline to 1.04 ± 0.612 mmol/kg/day, significantly reduced the prevalence of metabolic acidosis to 23.33% cases at the follow-up. Improvement in serum bicarbonate level showed significant dietary, anthropometric, and nutritional improvements in these patients. Hence, we conclude that correction of metabolic acidosis with optimal oral bicarbonate supplementation plays a pivotal role in the treatment of malnourished CKD patients on MHD.
Keywords
Hemodialysis
malnutrition
metabolic acidosis
Introduction
In health, protein and amino acids remain in equilibrium, however in the chronic kidney disease (CKD) patients, this balance is disturbed.[1] The progressive loss of kidney function is accompanied by metabolic acidosis because of the reduced capacity of the kidney to synthesize ammonia and excrete hydrogen ions.[2] Metabolic acidosis, if uncorrected, has been considered to have further deleterious effects on bone health and protein balance.[34] It is known to cause negative nitrogen balance, increased protein degradation, increased essential aminoacid oxidation, reduced albumin synthesis, and low appetite.[35] This eventually leads to decreased protein intake, protein energy malnutrition, loss of lean body mass, and muscle weakness.[5] Malnutrition is a frequently (5–70%) encountered problem in the hemodialysis (HD) patients.[6] With the advent of various clinical trials and guidelines, it has been widely agreed that the correction of metabolic acidosis should be an important goal of both the conservative management of CKD as well as dialysis therapy.[78] Oral bicarbonate supplementation is often required to correct acidosis. The correction of metabolic acidosis may have an impact on nutrition as measured by albumin and other markers of nutrition.[9] The relationship between correction of metabolic acidosis and nutritional status, as well as the optimal dosage of oral bicarbonate required to maintain serum bicarbonate level within the physiological range has not been studied in the Indian CKD population who are on maintenance HD (MHD).
Methods
This is a single-center prospective study conducted on 35 patients on MHD with a follow-up of 3 months at Dr. D.Y. Patil Medical College, Pune, in the Western part of India. Patients, receiving outpatient MHD for more than 1 month, were included and those with any acute illness were excluded. The Fresenius machine (4008 B and 4008 S) with the standard dialysate bicarbonate concentration of 35 mmol/L was used for HD. Each session was of 4 h with a blood flow rate of 300–400 ml/min and the dialysate flow rate of 500 ml/min. 29 out of 35 (82.9%) patients received twice weekly dialysis. The prerecruitment MHD schedule was kept unchanged during the entire study period.
After obtaining the written consent for their participation, the data on demographics, HD schedule, and the ongoing medications were noted. A detailed clinical and anthropometric examination including the height, weight, body mass index (BMI), waist hip ratio (WHR), mid arm circumference (MAC), triceps skin fold thickness (TSFT), and mid arm muscle thickness (MAMT) was carried out along with the laboratory investigations including hemogram, venous blood gas (VBG), urea, creatinine, serum electrolytes, serum total protein, and albumin at the baseline and at the follow-up after 3 months. The dietary assessment was done for consecutive 7 days with the diet diary at the baseline and at the follow-up. Metabolic acidosis and malnutrition in these patients were defined as a mid-week predialysis serum bicarbonate level <22 mmol/L and a BMI of <20 kg/m2, in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) 2012[10] and the EBPG (2007)[11] guidelines, respectively. The oral sodium bicarbonate dosage was increased in those patients whose baseline serum bicarbonate level was <22 mmol/L. The correlation between the change in oral sodium bicarbonate supplementation and the change in serum bicarbonate level, albumin level, and dietary parameters was assessed using the Pearson's correlation coefficient equation.
Results
As shown in Table 1, the baseline demographic data, the mean age of the study population was 47.54 ± 13.71 years. Twenty seven out of 35 (77.1%) cases were male and the rest were female, with a male:female ratio of 3.3. The mean duration of MHD was 18 ± 16.68 months. Chronic tubulo-interstitial disease in 13 out of 35 (37.1%) cases followed by diabetic nephropathy in 9 (25.7%) cases were among the most common causes of CKD in this cohort. Twenty nine out of 35 (82.9%) were on twice a week dialysis schedule, four (11.4%) cases were on once a week, and two (5.7%) cases were on thrice a week dialysis schedule. The study population had a mean pre- and post-HD serum creatinine of 8.21 ± 2.79 and 3.90 ± 2.14 mg%, respectively, whereas the mean pre- and post-HD blood urea was 101.94 ± 27.04 and 37.23 ± 18.50 mg%, respectively, at the baseline. The mean calculated urea reduction ratio (URR) and estimated Kt/V was 62.85 ± 15.74 and 1.17 ± 0.40, respectively, as shown in Table 1.

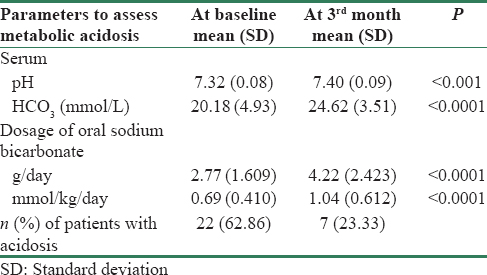
A total of 5 out of 35 patients were considered as dropouts, since three (8.6%) patients died and two (5.7%) patients were lost to follow-up during the study period. Table 2 shows the comparative parameters for the assessment of metabolic acidosis in this cohort, wherein the mean value of serum pH and HCO3 at the baseline evaluation was 7.32 ± 0.08 and 20.18 ± 4.93 mmol/L, respectively. Metabolic acidosis in accordance with KDIGO, 2012,[10] was found in 22 out of 35 (62.86%) patients in this cohort. The increments in the dosage of oral sodium bicarbonate supplements from 0.69 ± 0.410 to 1.04 ± 0.612 mmol/kg/day significantly reduced the prevalence of metabolic acidosis at the follow up, which was noted in only 7 out of 30 (23.33%) cases versus 22 of 35 (62.86%) cases at baseline.
As shown in the nutritional assessment [Table 3], significant dietary improvements were noted in the daily mean calorie intake which increased from 20.68 ± 6.49 to 21.06 ± 6.47 kcal/kg/day (P < 0.005) whereas the daily mean protein intake increased from 0.37 ± 0.13 to 0.41 ± 0.13 g/kg/day (P < 0.005). Furthermore, improvements were also noted in BMI from 18.81 ± 2.98 at baseline to 19.12 ± 2.96 kg/m2 (P < 0.001) at the follow-up.

Figure 1a–d show the co-relationship between changes in biochemical and dietary parameters. There was a moderate positive correlation noted between the change in oral bicarbonate supplementation and the rise in serum bicarbonate level (r = 0.56, P < 0.001). The change in dietary protein intake has a moderate positive correlation with the change in serum albumin levels (r = 0.41, P < 0.03).
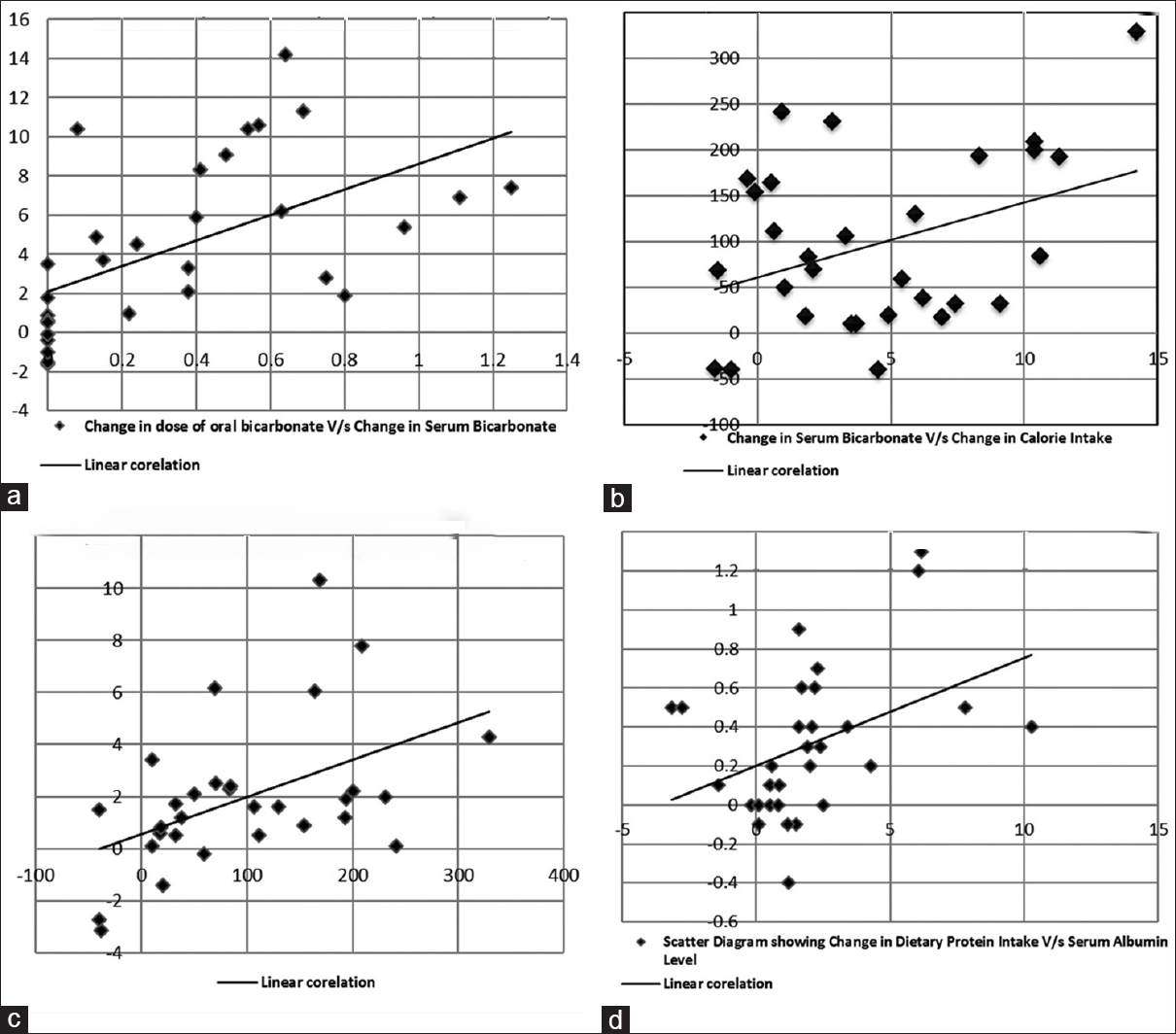
- (a) Scatter diagram showing change in dose of oral bicarbonate versus change in serum bicarbonate. (b) Scatter diagram showing change in serum bicarbonate versus change in calorie intake. (c) Scatter diagram showing change in calorie Intake versus change in protein intake. (d) Scatter diagram showing change in dietary protein intake versus change in serum albumin level
Discussion
Although the effects of metabolic acidosis on the human physiology were studied as early as in 1966 by Lemann et al.,[12] it was only in the mid-1980s that the detailed studies on metabolic acidosis in CKD patients gained momentum. Shortly thereafter in 1993, Oettinger and Oliver [3] noted that metabolic acidosis is common in patients with CKD because of the inability of the kidneys to excrete the nonvolatile acid load. Among the many goals of dialysis, the role in the correction of uremic acidosis was recognized. It was noted that despite adequate hours of HD, metabolic acidosis remains common and is reported in up to 75% of patients.
Out of 35 study subjects at the baseline evaluation, metabolic acidosis was found in 22 (62.86%) cases, in accordance with the KDIGO, 2012,[10] guidelines. As shown in Table 2, the mean value of serum HCO3 being 20.18 ± 4.93 mmol/L. We also noted that these patients were already receiving 2.77 ± 1.609 g/day, equivalent to 0.69 ± 0.410 mmol/kg/day, of oral sodium bicarbonate supplementation prior to their recruitment in this study. This signifies the prevalence of metabolic acidosis in this cohort. In the baseline dietary assessment, as shown in Table 3, the daily mean calorie and protein intake was 20.68 ± 6.49 kcal/kg/day and 0.37 ± 0.13 g/kg/day, respectively. This cohort had a mean calculated URR and estimated Kt/V of 62.85 ± 15.74 and 1.17 ± 0.40, respectively.
We tried to assess the possible reasons for low levels of serum bicarbonate in these patients. It could either be due to inadequate dialysis, poor nutrition, and/or inadequate oral bicarbonate supplementation. With the reference to EBPG (2007),[11] the patients from our center had a very low calorie and protein intake. Low-serum bicarbonate level in the presence of low protein intake indicates either inadequate dialysis or inadequate dose of oral sodium bicarbonate supplementation. Due to the financial constraints, low socioeconomic status, lack of education, and nonavailability of MHD center near the residence along with logistic problems, very few people in the Indian population receive adequate hours of HD, that is, 12 h/week or 4 h × 3 sessions of HD/week. At our center, most of the patients received 4 h × 2 sessions of HD/week as shown in Table 1. Hence, we continued the same (prerecruitment) schedule of HD of all the patients during this study, but changed the dosage of oral sodium bicarbonate on an individual basis.
After 3 months of modified dosage of oral sodium bicarbonate supplementation, we found that the levels of serum bicarbonate were significantly improved. The mean dose of oral sodium bicarbonate supplements as shown in Table 2, at baseline 0.69 ± 0.410, was increased to 1.04 ± 0.612 mmol/kg/day. These modifications significantly reduced the prevalence as well as severity of metabolic acidosis in the study population, metabolic acidosis was found only in 7 out of 30 (23.33%) cases at the follow-up as against 22 of 35 (62.86%) cases at baseline. The VBG revealed a significant improvement in the mean pH and mean bicarbonate from 7.32 ± 0.08 to 7.40 ± 0.09 (P < 0.001) and 20.18 ± 4.93 to 24.62 ± 3.51 mmol/L (P < 0.0001), respectively. Hence, we conclude that the adequate dosage of oral sodium bicarbonate supplementation improves serum bicarbonate level.
The dietary protein and calorie intake in this cohort at follow-up showed a statistically significant improvement. As shown in Table 3, the daily mean calorie intake increased from baseline 20.68 ± 6.49 to 21.06 ± 6.47 kcal/kg/day (P < 0.005) at follow up, and the daily mean protein intake increased from 0.37 ± 0.13 to 0.41 ± 0.13 g/kg/day (P < 0.005), respectively. Further, there was a statistically significant improvement in the mean dry weight from 49.37 ± 9.45 Kg at baseline to 50.17 ± 9.56 kg (P < 0.0001) at 3rd month. The BMI also improved from 18.81 ± 2.98 kg/m2 at baseline to 19.12 ± 2.96 kg/m2 at 3rd month (P < 0.001). Similarly, significant improvement was also noted in the anthropometric parameters such as mean MAC, which increased from 22.57 ± 4.75 cm at baseline to 23.90 ± 3.79 cm at follow-up (P < 0.01). The mean TSFT decreased from 1.08 ± 0.38 to 0.88 ± 0.31 cm (P < 0.05). The changes in the mean WHR from 0.94 ± 0.09 to 0.94 ± 0.08 (P > 0.05) and MAMT from 25.57 ± 5.15 cm to 26.27 ± 4.33 cm (P > 0.05) were not statistically significant.
The laboratory assessment at the follow-up after 3 months showed significant improvement in the serum albumin level, which is a marker of nutritional status in accordance with the EBPG,[11] as well as the KDIGO, 2012,[10] guidelines. As shown in Table 3, though the mean serum total protein did not show any statistically significant improvement, the mean serum albumin significantly improved from the baseline value of 3.87 ± 0.45 to 4.17 ± 0.36 g% (P < 0.0001) at the follow-up.
We further tried to assess and correlate the change in serum bicarbonate levels with the change in oral bicarbonate supplementation to determine the optimal dosage required to increase the serum bicarbonate levels near the physiological range. As shown in Figure 1a, we found that there was a moderate positive correlation between the change in the dosage of oral sodium bicarbonate and the rise in serum bicarbonate level (r = 0.56, P < 0.001). We also noted that increments in serum bicarbonate levels improved dietary calorie intake with a moderate positive correlation between the two (r = 0.37, P < 0.04), and that the change in dietary calorie intake correlated with the change in dietary protein intake, which again was significant statistically (r = 0.48, P < 0.008) as shown in Figure 1b and c. Furthermore, the change in dietary protein intake showed a statistically significant positive correlation with the change in serum albumin levels (r = 0.41, P < 0.03), as shown in Figure 1d. Thus, increasing the dosage of oral sodium bicarbonate supplementation not only improved metabolic acidosis, but also the nutrition of this cohort.
The present study is comparable to the study conducted by Movilli et al.,[13] since both are prospective cohort studies undertaken on chronic stable HD patients with the aim to study the role of oral sodium bicarbonate supplementation in MHD population. Movilli et al.[13] had concluded that correction of metabolic acidosis in HD patients improved serum albumin levels and further that in the presence of moderate to severe acidosis, nPCR does not reflect the real dietary protein intake of these patients, probably due to increased catabolism of endogenous proteins. The findings of the present study match the findings of the previous study in terms of improvement in serum bicarbonate and serum albumin levels, even though there were variations in the methodologies of the two studies. In their study, they had defined metabolic acidosis as serum bicarbonate values <20 mmol/L and also had studied the role of nPCR. The differences were also noted in the schedule of HD, which was three times a week for all the patients in the previous study, whereas in the present study, most of the patients were on twice a week schedule, barring the exception of two cases on thrice weekly and four cases on once weekly MHD. In spite of these differences, the results and conclusions of both the studies are similar showing significant improvements in serum bicarbonate and albumin levels after treatment with adequate dosage of oral sodium bicarbonate supplementation.
Soleymanian and Ghods [14] in Iran studied the deleterious effects of metabolic acidosis on nutrition of 47 anuric HD patients. They noted a statistically significant direct correlation between serum albumin and BMI. In the present study, we could not establish such a correlation between serum albumin and BMI. However, a significant positive correlation was noted between the change in bicarbonate dose and the calorie and protein intake. Increased protein intake was found to have a statistically significant positive correlation (r = 0.41, P < 0.03) with serum albumin levels.
In both these studies, the patients received more dialysis than our study population. This highlights the importance of optimal oral bicarbonate supplementation in the inadequately dialyzed and poorly nourished Indian HD patients. With a 52.3% increase in the mean oral bicarbonate dose, the percentage of patients with metabolic acidosis reduced from 62% to 22%. This dose was 4.22 g/day (19.3 mmol/day, 1.04 mmol/kg/day).
Conclusion
Therefore, we conclude that there is a significant prevalence of metabolic acidosis and malnutrition in the Indian patients of CKD on MHD. Optimal dosage of oral bicarbonate supplementation is of paramount importance in correcting metabolic acidosis in the HD patients. Most probably, the dose of 1 mmol/kg/day of oral bicarbonate would keep almost 78% patients free from metabolic acidosis. The improvement in metabolic acidosis is also associated with improvement in nutrition and the clinical and biochemical markers of nutrition.
Financial support and sponsorship
Dr. D. Y. Patil Medical College, Hospital and Research Centre (DPU), Pune - 411 018.
Conflicts of interest
There are no conflicts of interest.
References
- Metabolic acidosis and uremic toxicity: Protein and amino acid metabolism. Semin Nephrol. 1994;14:232-7.
- [Google Scholar]
- Correction of chronic metabolic acidosis for chronic kidney disease patients. Cochrane Libr. 2009;3:1-32.
- [Google Scholar]
- Normalization of uremic acidosis in hemodialysis patients with a high bicarbonate dialysate. J Am Soc Nephrol. 1993;3:1804-7.
- [Google Scholar]
- Metabolic acidosis in maintenance dialysis patients: Clinical considerations. Kidney Int Suppl. 20035;88:S13-25.
- [Google Scholar]
- Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl. 2005;95:S21-7.
- [Google Scholar]
- Factors causing catabolism in maintenance hemodialysis patients. Miner Electrolyte Metab. 1992;18:280-3.
- [Google Scholar]
- Correction of metabolic acidosis to ameliorate wasting in chronic kidney disease: Goals and strategies. Semin Nephrol. 2009;29:67-74.
- [Google Scholar]
- What should define optimal correction of metabolic acidosis in chronic kidney disease? Semin Dial. 2010;23:411-4.
- [Google Scholar]
- A review of the effects of correction of acidosis on nutrition in dialysis patients. Semin Dial. 2000;13:252-5.
- [Google Scholar]
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
- [Google Scholar]
- The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45:1608-14.
- [Google Scholar]
- Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: A prospective study. Nephrol Dial Transplant. 1998;13:1719-22.
- [Google Scholar]
- The deleterious effect of metabolic acidosis on nutritional status of hemodialysis patients. Saudi J Kidney Dis Transpl. 2011;22:1149-54.
- [Google Scholar]







