Translate this page into:
The spectrum of glomerular diseases in a single center: A clinicopathological correlation
Address for correspondence: Dr. Vishal Golay, Department of Nephrology, IPGMER and SSKM Hospital, 242, AJC Bose Road, Kolkata, West Bengal- 700 020, India. E-mail: rvgolay@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We report the spectrum of biopsy-proven glomerular disease (GD) in a single center in Eastern India. Medical records of 666 patients with biopsy-proven GD over a period of 2 years from July 2010 to July 2012 were retrospectively analyzed. The clinical, laboratory, and histological data were recorded. All biopsy specimens were examined by the same pathologist with light and immunofluorescence microscopy. Electron microscopic analysis was performed only in selected cases. Histologic spectrum of various GDs was studied along with its correlation with the clinical and laboratory parameters. The clinical diagnosis was nephrotic syndrome (NS) in 410 (61.56%), rapidly progressive renal failure/glomerulonephritis in 130 (19.52%), subnephrotic proteinuria/asymtomatic urinary abnormalities in 52 (7.81%), acute kidney injury/acute nephritic syndrome in 40 (6.01%), and macroscopic hematuria in 4 (0.6%) patients. Male: Female ratio was 1.05; 27.92% (n = 186) were < 18 years, 68.47% (n = 456) were 18–59 years, and 3.6% (n = 24) were ≥ 60 years of age. The most common GD was minimal change disease (MCD) (20.12%, n = 134); others were focal segmental glomerulosclerosis (FSGS) (18.02%, n = 15.32%), lupus nephritis (LN) (15.32%, n = 102), membranous nephropathy (MN) (12.01%, n = 80), and IgA nephropathy (IgAN) (8.11%, n = 54). Primary GD was present in 79.13% (n = 527) and common histologies were MCD (25.42%), FSGS (22.58%), MN (14.42%), and IgAN (10.25%). Secondary GD was present in 20.87% (n = 139), with the most common being LN (73.38%, n = 102). Among the NS (n = 410), the most common GD was MCD (31.46%), followed by FSGS (25.6%), MN (15.58%), LN (7.8%), IgAN (6.09%), and membranoproliferative glomerulonephritis (4.88%). FSGS was the most common primary GD in adults, MCD in children, and MN in the elderly patients. The spectrum of GD varies according to the area of study and changes over time. A biopsy registry is needed for documenting this variation.
Keywords
Clinicopathologic correlation
glomerular disease
kidney biopsy
registry
spectrum
Introduction
Glomerular disease (GD) is one of the most common forms of renal diseases and can have many different clinical presentations. It can present as nephrotic syndrome (NS), nephritic syndrome, rapidly progressive renal failure (RPRF), acute kidney injury (AKI), chronic kidney disease (CKD), macroscopic hematuria (MH), recurrent disease in the posttransplant kidney, as well as isolated proteinuria or hematuria.[1] In any case, a kidney biopsy is needed for the correct characterization of various types of GD.
Biopsy registries as it can give an idea of the regional variations in the spectrum of GD as well as the trend over time. In one of the largest reports of 6469 biopsies with GD from the University of North Carolina, focal segmental glomerulosclerosis (FSGS) was the most common GD (14.22%) followed by membranous nephropathy (MN) (13.09%).[2] However, there is a variation in the prevalence of the type of GD according to geographical location and race of the study population. IgA nephropathy (IgAN) is the common primary GD is studies from East Asia,[3–5] as well as in white Europeans and Americans.[6–10] In contrast, FSGS is the most common GD among African-Americans, South Americans, and in the Middle East.[11–13]
The change in the spectrum of GD over the last few decades has been demonstrated in many studies worldwide, with most showing a trend toward increase in FSGS cases.[3671112] There are a limited number of studies from India and most of them are from Southern and Northern Indian centers.[13–16] These studies also demonstrate a trend toward increase in the incidence of FSGS[14–16] and a decrease in membranoproliferative glomerulonephritis (MPGN).[16] The incidence of non-IgA mesangial proliferative GN (MesPGN) was also quite high (20.2%) in one of these studies.[16]
Thus, there is a great variation in the presentation of GD across the globe and the disease spectrum has also been changing over the last few decades. In light of the paucity of data from Eastern India, we studied the prevalence of GD in a large tertiary care referral center in East India.
Materials and Methods
All the kidney biopsies that were performed in our institute over a period of 2 years from July 2010 to July 2012 were retrospectively analyzed. The clinical records of these patients were reviewed and their clinical diagnoses at presentation ascertained. Standard definitions were used for classifying the clinical syndromes.[17] Patients were classified into six categories: NS, acute nephritic syndrome (ANS)/AKI, RPRF/rapidly progressive glomerulonephritis (RPGN), MH, asymptomatic urinary abnormalities (AUA), and CKD. Patients with CKD were biopsied only if they did not have diabetes mellitus as the underlying cause, or if they were being worked up for a renal transplantation and had active urinary sediments with normal sized kidneys on ultrasonography (USG). All the baseline clinical details along with the relevant laboratory investigations were recorded. Majority of the patients had been worked up for secondary etiologies depending on the glomerular histology.
All biopsies were performed under real-time USG guidance using the Bard® Max-Core® Disposable Core Biopsy Instrument (Bard Biopsy Systems, USA). A 16 G × 16 cm size instrument was used for adults ≥ 18 years old, and a smaller 18 G × 16 cm instrument was used for those < 18 years of age. At least two cores were obtained and samples sent for light microscopy (LM) and immunofluorescence (IF) microscopy in all cases and for electron microscopy (EM) in select cases or where it could be afforded by the patient. LM was carried out using H and E, periodic acid-Schiff, Jones silver, and Trichrome stains. Additional special stains were used whenever indicated. IF staining was performed on 3-μm cryostat sections using polyclonal fluorescein isothiocyanate-conjugated (FITC) antibodies to IgG, IgM, IgA, C3, C1q, and kappa and lambda light chains (DakoCytomation, Denmark). The intensity of IF staining was graded on a scale of 0 to 3+. All renal biopsy examinations were confirmed by the same pathologist (A. Abraham.). If a biopsy sample was inadequate for diagnosis, a second biopsy was also performed, if exact diagnosis would have a significant bearing on the therapy and if the patients gave consent for a re-biopsy.
Glomerular pathologies were classified into the following: (a) Primary glomerular diseases (PGD); major ones being IgAN, FSGS, MN, MCD, MPGN, postinfectious glomerulonephritis (PIGN), MesPGN, crescentic glomerulonephritis (CresGN), and other rare ones such as thin basement membrane disease (TBMD), idiopathic nodular glomerulosclerosis, and C1q nephropathy. (b) Secondary glomerular diseases (SGD) which included lupus nephritis (LN), glomerulonephritis related to hepatitis B or C, systemic vasculitides, Henoch-Schönlein purpura, diabetic nephropathy, amyloidosis, light chain deposition disease (LCDD), Alport’s syndrome, and vascular nephropathies including benign and malignant nephrosclerosis and thrombotic microangiopathies. Patients with evidence of chronic glomerulosclerosis were also classified into PGD or SGD depending on the findings on histology. Interstitial fibrosis (IF) and tubular atrophy (TA) were graded as mild (<25%), moderate (25-50%), and severe (>50%) on LM.
Descriptive statistics was used and results were expressed as frequencies, percentages, and mean ± SD Spearman’s rank order correlation was used to determine the correlation between various serum creatinine categories (<1 mg/dL, 1-1.4 mg/dL, 1.5-3 mg/dL, and ≥ 3 mg/dL) and the extent of IF/TA (none, mild, moderate, and severe). Those with a clinical diagnosis of RPRF/RPGN and ANS/AKI were excluded from the correlation analysis. P value of < 0.05 was considered significant. Statistical analysis was carried out using IBM compatible SPSS for Windows ver. 10 (SPSS, Chicago, IL, USA).
Results
A total of 666 biopsy-proven GD were recorded over the period of study. LM and IF were performed in all biopsies and the average glomerular yield was adequate (28.84 ± 14.62 glomeruli). EM was performed only in 6.9% of biopsies. The average age of the patients was 28 ± 14.62 years and Male: Female ratio was 1.05 (341 males: 325 females). The contribution of the various glomerular syndromes to the performance of kidney biopsy is presented in Figure 1. The demographic and baseline characteristics of each of these glomerular syndromes are described in Table 1 and the various types of histology seen in each syndrome are presented in Table 2.
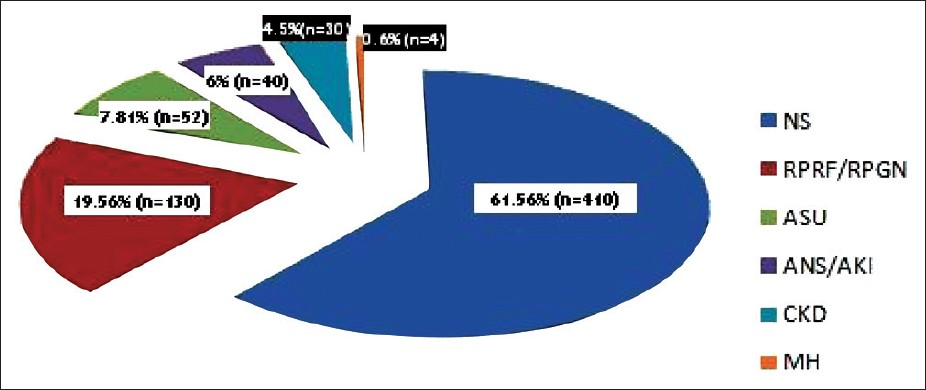
- Spectrum of various clinical syndromes


The most common GD presenting as NS was MCD (32.46%), and the other common ones being FSGS (25.12%), MN (16.09%), and IgAN (6.09%). LN was seen in 7.80% (n = 32) of NS and 43.75% of these were either pure class V LN or class V overlap with other LN classes. The most common etiology of RPRF/RPGN was LN seen in 33.85% (n = 44) of cases, 84.09% of them being class IV LN. The other common GD presenting as RPRF/RPGN was CresGN (26.15%, n = 34), of which 26 were pauci-immune CresGN (7 of them being anti-neutrophil cytoplasmic antibody (ANCA) associated), 7 were immune complex CresGN, and two were anti-glomerular basement membrane (GBM) disease. PIGN was the most common cause of ANS (37.5%, n = 15) [Table 2].
There was a slight male predominance except in the case of RPRF/RPGN and ANS/AKI which could be due to the greater contribution of LN to these syndromes. LN, MPGN, and CresGN were seen predominantly in female patients with a female-to-male ratio of 4.67:1, 1.5:1, and 1.38:1, respectively. IgAN, FSGS, MCD, and MN were seen more in male patients with a male-to-female ratio of 2.73:1, 2:1, 1.44:1, and 1.16:1, respectively [Table 3 and Figure 2].


- Gender-specific distribution of common glomerular disease
Overall, the most common GD was MCD (20.12%, n = 134). Of the total 666 cases, PGD was seen in 527 (79.13%) biopsies and the most common PGD was again MCD (25.42%, n = 134) closely followed by FSGS (22.58%, n = 119). SGD was seen in 135 (20.87%) biopsies and LN was seen in majority of the cases (73.38%, n = 102). The spectrum of GD along with the PGD and SGD is presented in Figure 3 and Table 4.

- Primary and secondary glomerular diseases

The GDs were also studied based on the serum creatinine values. They were divided into three groups of serum creatinine: <1.5 mg/dL, 1.5–3 mg/dL, and ≥ 3 mg/dL [Table 5]. GDs were also classified according to the age of presentation (<12 years, 12–17 years, 18–59 years, and ≥ 60 years [Table 6]). MCD was the most common GD in the age groups of < 12 and 12–17 years, whereas LN followed by FSGS was the most common GD in adults in age group 18–59 years. However, FSGS was the commonest PGD in adults and MN was the commonest GD in the elderly patients. In all, 49.25% of MCD was seen in patients < 18 years of age, whereas adults (18–59 years) contributed to 48.51% of MCD. There were two cases of MCD in the elderly patients and both of them had presented with raised serum creatinine levels and had evidence of acute tubular injury on biopsy. All the other GDs were most commonly seen in adults.


There were 112 biopsies showing crescents on LM. The diseases that had shown these crescents were CresGN (n = 40, 35.71%), LN (n = 35, 31.25%), PIGN (n = 11, 9.82%), IgAN (n = 10, 8.92%), MPGN (n = 6, 5.36%), and MN (n = 5, 4.46%). There were also three cases with advanced renal disease showing fibrous crescents. As expected, the disease with the severest changes was CresGN, with an average of 75.38 ± 25.66% crescents on biopsy. A total of 120 biopsies showed a FSGS pattern of histology and the most common type was the not otherwise specified (NOS) seen in 90 (80%) cases, followed by tip lesion in 13 (10.83%), perihilar in 7 (5.83%), and collapsing and cellular variants in 2 (1.67%) cases each. Both cases of FSGS collapsing variant were seen in kidney transplant recipients, presenting as NS.
A Spearman’s rank order correlation was run to determine the relationship between serum creatinine categories and the presence of TA and IF, categorized into none, mild, moderate, and severe as defined. There was a positive correlation between serum creatinine and the extent of IF/TA, which was statistically significant (ρ =0.542, P = 0.001).
Discussion
This study is a single-center experience in India and is restricted to the last 2 years only and is the latest data of GD from the subcontinent. We were unable to analyze the data for the period before this due to inadequate data and poor standardization of the biopsy reporting. Another shortcoming of our study is the inability to perform EM in all cases which would have helped in better diagnosis. However, we feel that a relatively accurate diagnosis could be achieved in a majority of cases.
There are regional variations as well as change in the spectrum of presentation of GD over time as is seen in registry data from various parts of the world. Although a national biopsy registry is not existent in India and the neighboring countries, there are some published data. Table 7 summarizes data from India and the neighboring countries. As is clear from this table, even among studies originating from the regional centers, there is a variation in presentation of various GDs.

The main indication of a kidney biopsy for GD was NS in our study, which was seen in 61.56% of the patients. However, as we had included only cases with glomerular involvement in this study, the actual contribution of NS in the total number of kidney biopsies would be lower and would be comparable to other studies where the contribution of NS to kidney biopsies ranged from 34% to 60%.[3912141518–21] AUA contributed to only 7.8% of biopsies in our study, which similar to that in the studies published from India but is much less compared to that in the studies from other parts of the world. This could be due to the local practice of routine screening or greater acceptance of performance of renal biopsies for evaluation of AUA.
The incidence of MesPGN is much lower in our data (0.6%) compared to that in the other regional studies.[14–16] One reason of this could be the fact that these studies include older data. Improvement in IF technique, reporting, and interpretation over the last decade could have led to lesser diagnosis of non-IgA MesPGN. Even in the comparative analysis among biopsies performed in the same center over a period of time, one of the studies showed a decline in the prevalence of MesPGN.[15] This decline over time was also seen in studies from other parts of the world.[39] This was, however, not seen in another study from South India.[14] Another reason for this decline could be that many of the older studies did not evaluate all biopsies with IF compared to the universal use of IF in all biopsies that are currently performed.[2122]
The most common etiology of GD in our study across all ages was MCD (20%) and it remained to be the most common cause of PGD as well (25.42%). However, FSGS was the most common PGD in adults (≥18 years) and MN was most common in the elderly patients (≥60 years). Das et al., from India also showed that MCD was the most common PGD across all ages including adults.[14] MCD was also the commonest GD in a study from North India.[23] But in another study from South India, Balakrishnan et al., showed that FSGS is the most common GD among the adults.[15] The most prevalent form of GD varies across the world, with IgAN being the most common form in some and FSGS being common in some, as already described. It should also be noted that in the two large series from India, the trend was toward an increase in the prevalence of FSGS.[1415]
FSGS-NOS was the most common FSGS variant in our study (80%), followed by tip lesion in 10.83%, perihilar in 5.83%, and collapsing and cellular variants in 1.67% each. A similar spectrum was reported in a study of 210 biopsies of FSGS from India, where the NOS variant was seen in 72.5%, and tip lesion, cellular, perihilar, and collapsing variants were seen in 13.5%, 8%, 4%, and 2%, respectively.[23] The spectrum of FSGS is different in studies from other countries, with the NOS variant still being the commonest but much lesser than that seen in the Indian studies.[24–27] In these studies, the other variants also differ depending on the racial contribution and patient selection.
In our study, IgAN was seen only in 8.11% of all cases. IgAN was reported in 5.02%[14] and 9.13%[15] from two other studies from South India. The prevalence of IgAN seems to be much lower in data originating from North India and the neighboring countries of Pakistan and Nepal.[13182829] This could be due to factors such as the different racial stock of population residing in these parts or simply due to technical reasons. Across the globe IgAN is seen quite frequently, being the most common form of GD in studies originating from East Asia,[3–5] as well as in white Europeans and Americans.[6–10] The greater prevalence of IgAN in the developed countries could be due to greater population screening and the following biopsy in patients with AUA. However, even in the large report from China where such screening program was not undertaken, IgAN was the most prevalent PGD being present in 54.3% of all cases, and it remained the commonest form of PGD across all ages except in those ≥ 60 years old.[4] This could again very well be due to racial factors as the indications for renal biopsy was similar in studies where IgAN was very prevalent and in those where the prevalence was lesser.
LN was the most common SGD in our study seen in 73.38% and it is uniformly the commonest cause of SGD worldwide. Amyloidosis was seen in 8 (1.2%) cases, 5 of them were secondary amyloidosis and 3 were amyloid light-chain (AL)-amyloidosis presenting as LCDD. This is comparable to other studies from other Indian centers.[13–15] This prevalence is lower than that seen in studies from other countries where amyloidosis contributed to 2.5–3% of cases.[7930] In contrast, there was a high prevalence (4.6% of all GD) of amyloidosis (mostly secondary) in the study from Pakistan.[18] This was attributed by the authors to the high endemicity of tuberculosis in their study population. The low prevalence of amyloidosis in our study as well as in studies from other centers in India could represent a regional trend or could be a referral bias wherein timely evaluation for renal disease in patients with other chronic illnesses and referral to higher centers were not carried out by primary care physicians.
Conclusion
It seems that FSGS is fast becoming the most common GD, especially among adults in the Indian population, and there is also a decline in the incidence of IgA-negative MesPGN. IgAN is not very prevalent in the Indian population compared to other parts of the World and is still less prevalent in Northern India. It is also to be noted that although EM was performed in only 6.9% of biopsies, we had one case of TBMD and Alport’s syndrome each. Thus widespread use of EM in all biopsies will go a long way in detecting rarer forms of GD; but for the meantime, a large proportion of centers have to manage without it. As India is a racially heterogenous country, a national biopsy registry data should be established to address these regional differences in the spectrum of GD.
Acknowledgments
We thank all the residents and the nursing staff of the Department of Nephrology who have played a role in performing the biopsies as well as taking care of the patients.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Primary glomerular disease. In: Brenner BM, ed. Brenner and Rector’s The Kidney (8th ed). Philadelphia: Saunders; 2008. p. :1101-91.
- [Google Scholar]
- Glomerular clinicopathologic syndromes. In: Greenberg A, Cheung AK, Coffman TM, Falk RJ, Jennette JC, eds. Primer on Kidney Diseases (5th ed). Philadelphia: Saunders; 2009. p. :148-59.
- [Google Scholar]
- The changing spectrum of primary glomerular diseases within 15 years: A survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant. 2009;24:870-6.
- [Google Scholar]
- Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406-10.
- [Google Scholar]
- Trends in the incidence of biopsy-proven glomerular diseases in the adult population in Central Poland in the years 1990-2010. Kidney Blood Press Res. 2012;35:254-8.
- [Google Scholar]
- Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483-7.
- [Google Scholar]
- The Italian experience of the national registry of renal biopsies. Kidney Int. 2004;66:890-4.
- [Google Scholar]
- The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant. 2004;19:3040-9.
- [Google Scholar]
- The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant. 2009;24:3050-4.
- [Google Scholar]
- Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000;35:878-83.
- [Google Scholar]
- An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant. 2010;25:490-6.
- [Google Scholar]
- Prevalence of glomerular diseases: King Khalid University Hospital, Saudi Arabia. Saudi J Kidney Dis Transpl. 2000;11:442-8.
- [Google Scholar]
- Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol. 2011;21:250-7.
- [Google Scholar]
- Spectrum of biopsy proven renal disease and changing trends at a tropical tertiary care centre 1990-2001. Indian J Nephrol. 2003;13:29-35.
- [Google Scholar]
- Characterization of kidney lesions in Indian adults: Towards a renal biopsy registry. J Nephrol. 2006;19:205-10.
- [Google Scholar]
- Introduction to glomerular disease: Clinical presentations. In: Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology (4th ed). St. Louis, Missouri: Saunders; 2010. p. :193-207.
- [Google Scholar]
- Etiological profile of nephrotic syndrome in Kashmir. Indian J Nephrol. 2008;18:9-12.
- [Google Scholar]
- Hisopathological spectrum of glomerular disease in Nepal: A seven-year retrospective study. Nepal Med Coll J. 2008;10:126-8.
- [Google Scholar]
- The changing pattern of glomerulonephritis in Singapore over the past two decades. Clin Nephrol. 1999;52:96-102.
- [Google Scholar]
- The epidemiology and prognosis of glomerulonephritis in Denmark 1985-1997. Nephrol Dial Transplant. 1999;14:1889-97.
- [Google Scholar]
- Patterns of renal disease in Cape Town South Africa: A 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant. 2011;26:1853-61.
- [Google Scholar]
- Primary focal segmental glomerulosclerosis in adults: Is the Indian cohort different? Nephrol Dial Transplant. 2009;24:3701-7.
- [Google Scholar]
- Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int. 2006;69:920-6.
- [Google Scholar]
- Clinicopathologic study of different variants of focal segmental glomerulosclerosis. Zhonghua Bing Li Xue Za Zhi. 2007;36:11-4.
- [Google Scholar]
- Pathological variants of focal segmental glomerulosclerosis in an adult Dutch population - Epidemiology and outcome. Nephrol Dial Transplant. 2008;23:186-92.
- [Google Scholar]
- Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrology. 2011;16:87-92.
- [Google Scholar]
- Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Nephron. 1999;82:205-13.
- [Google Scholar]
- Presenting features and short-term outcome according to pathologic variant in childhood primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2:700-7.
- [Google Scholar]







