Translate this page into:
Thrombotic microangiopathy in renal allografts
Address for correspondence: Dr. Radha Sistla, Plot No. 20, Road No. 1, Alakapuri, Hyderabad - 500 035, India. E-mail: sradha_21@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Thrombotic microangiopathy (TMA) is a serious complication of renal transplantation. It is a morphological expression of various etiological factors. In a renal allograft, TMA can occur de novo or be a recurrent disease. The aim of this study was to analyze the etiological factors and observe the changing trends of TMA with respect to emerging new etiological factors. We evaluated 131 graft biopsies over a period of 2½ years (2010-2012). All the renal biopsies were formalin fixed, paraffin embedded. Twenty serial sections were studied. Stains routinely used were Hematoxylin and Eosin, Periodic Acid Schiff, Massons Trichrome and Silver Methenamine stains. C4d by immunohistochemical method was done on all graft biopsies. Incidence of TMA in our series was 9.1%. Out of the 12 cases, five were associated with calcineurin inhibitor toxicity, three were diagnosed as acute antibody-mediated rejection, and two were recurrent haemolytic uremic syndrome. One patient developed haemolytic uremic syndrome on treatment with sirolimus and one patient was cytomegalovirus positive on treatment with ganciclovir, developed haemolytic uremic syndrome during treatment course. This study describes a spectrum of etiological factors for thrombotic mciroangiopathy ranging from common cause like calcineurin inhibitor toxicity to rare cause like ganciclovir induced TMA.
Keywords
Etiologies
recurrent and de novo thrombotic microangiopathy
renal allografts
Introduction
Thrombotic microangiopathy (TMA) is being reported with increasing frequency. TMA is a morphological lesion characterized by platelets and fibrin thrombi seen in the vasculature causing acute renal failure in immediate post-transplant period. Various etiological factors are associated with development of TMA in a renal transplant setting. TMA can occur as a recurrent or De novo disease. De novo disease is associated with calcineurin inhibitors, mTORs, acute humoral rejection and various viral infections. The association of cyclosporine with TMA was first described. in 1991.[1] With increase in use of tacrolimus, tacrolimus associated TMA is on the rise.[2] Clinical presentations vary from renal limited disease to systemic disease.
Subjects and Methods
A total number of 131 graft biopsies were analyzed over a 2½ year period from 2010 to 2012. All the cases of TMA diagnosed in graft biopsies were included in the study. Inadequate biopsies and biopsies with extensive cortical necrosis without viable areas were excluded. TMA was diagnosed in 12 cases and constituted 9.1% of the biopsies. The age ranged from 10 years to 52 years. There were 11 male patients and one female patient. Renal biopsy for light microscopy was formalin fixed, paraffin embedded and three micron thin sections were cut. A total of 20 sections were studied, stained with Hematoxylin and Eosin (H and E), Periodic Acid Schiff (PAS), Silver Methenamine and Masson's Trichrome in each case. C4d was done on all graft biopsies as a part of protocol for evaluation of graft biopsies by immunohistochemistry. Histological findings included mesangiolysis, splitting of basement membrane, endothelial swelling and thrombi in capillary lumina. Extraglomerular vessel showed thrombi and there was cortical infarction in two cases. C4d was positive in three cases, donor specific antibodies were done in oneof these three cases and was positive.
Duration of biopies ranged from 48 h post-transplant day to the 10th month after transplant. Serum creatinine levels ranged from 1.8 mg/dl to 9 mg/dl. There was correlation with tacrolimus trough levels in two of the three cases associated with tacrolimus toxicity. Two cases were recurrent disease.
Results
Twelve cases of TMA were diagnosed out of 131 graft biopsies studied over a period of 2½ years. Two cases were recurrent disease and ten cases occurred De novo in renal transplants [Table 1]. Male patients were predominant with a M:F ratio of 11:1. Two cases diagnosed as recurrent disease had native disease of haemolytic uremic syndrome. Disease was not active at the time of transplantation. Factor deficiencies were not investigated. Both the donors were live related, one received from paternal aunt and other from his wife. Graft dysfunction in these patients was associated with haematological changes like hemolysis and thrombocytopenia. There was graft loss in both the patients and they were put back on dialysis. Three patients developed renal limited De novo TMA while on treatment with calcineurin inhibitor-Tacrolimus. Out of these three patients, two were found to have high trough levels of tacrolimus. In these two patients the tacrolimus levels were adjusted and subsequently they showed improvement in graft function. In one patient who had associated toxic epidermal necrolysis, tacrolimus was stopped and patient was switched to sirolimus. This patient subsequently developed sepsis and died. Two cases were due to cyclosporine toxicity. Both patients had renal limited disease and with change in the immunosuppressive protocol subsequently recovered graft function.

Out of the 12 cases, three cases were diagnosed as acute humoral rejection. C4d was positive in all these three cases. One patient presented with graft dysfunction in the immediate post-transplant period (48 h). Disease was extensive in this patient with histological features of cortical necrosis. It was an ABO compatible graft, pre transplant cross match was negative. Cross matching was done by flow cytometric method. Inspite of five sessions of plasmapheresis, there was graft loss. Graft nephrectomy was done and patient was put back on renal replacement therapy. In the other two patients, one patient showed improved graft function with plasmapheresis and antithymocyte immunoglobulin. The other patient had a graft loss.
One case TMA was noted in a patient whose immuno suppression was a calcineurin inhibitor free regimen. Regimen included sirolimus, mycophenolate mofetil and steroids. C4d was negative in this patient. The etiological factor for TMA in this case was concluded to be sirolimus induced.
One patient developed cytomegalovirus disease three months after transplant. During treatment with ganciclovir, patient developed graft dysfunction. Graft biopsy revealed features of TMA. There were no systemic symptoms of TMA. H and E sections did not reveal any intranuclear inclusions. Immunohistochemical stains for cytomegalovirus was not done.
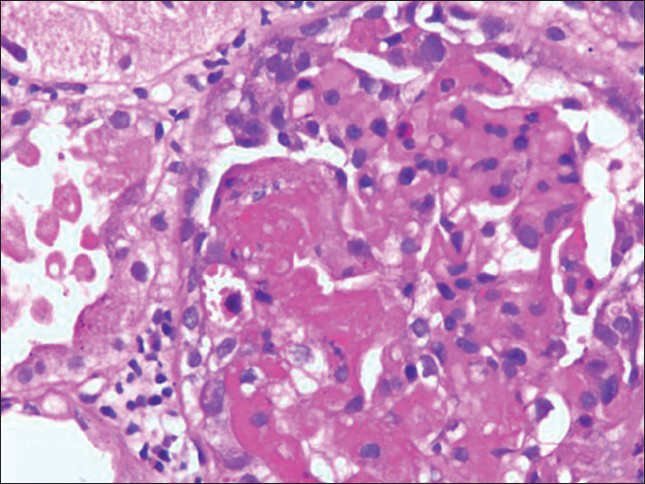
TMA observed istological features included mesangiolysis, duplication of basement membrane, endothelial swelling and fibrin thrombi in glomerular capillaries. [Figure 1a and b] Arterioles also showed fibrin thrombi in the lumina. Small arteries revealed necrosis of wall with thrombus formation.
TMA secondary to acute humoral rejection in addition had endothelitis, [Figure 2] capillaritis with positive C4d staining [Figure 3] and cortical necrosis [Figure 4].

- (a) Glomerulus showing fibrin thrombi and mesangiolysis. (H and E ×100), (b) Fibrin thrombi in glomerulus. (Massons trichrome ×400)
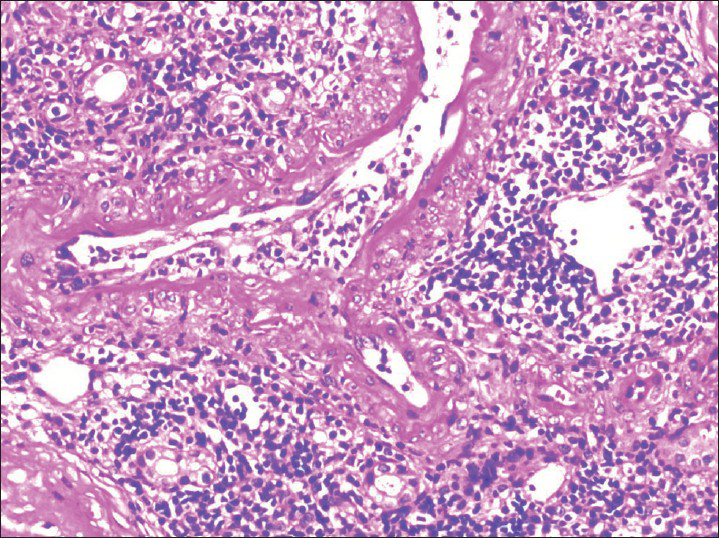
- Vessel showing endothelitis in rejection (H and E, ×200)

- Positive C4d staining in peritubular capillaries (×200)
- Cortical necrosis in acute humoral rejection (H and E, ×200)
Patients presented with graft dysfunction as early as the second day of post-transplant period to the 10th month.
Five patients had graft loss and had to be put on renal replacement therapy. Graft function recovered in six patients and these patients are apparently doing well till date.
Discussion
TMA is a serious complication in renal transplant recipients. It is a histopathological term that is used to define glomerular, arteriolar and arterial lesions with patchy involvement. It is characterized by mesangiolysis, fibrin thrombi, splitting of capillary basement membrane and cortical necrosis. Two forms of post-transplant TMA are described, recurrent disease and De novo TMA. The reported incidence of De novo TMA is variable in various studies, the incidence quoted is 4-14%.[1] Incidence of TMA in our series is 9.1%. There are various etiological factors for TMA in renal grafts like Cytomegalovirus infection,[2] Parvovirus B19 infection,[3] BK Polyoma nephritis,[4] antiphospholipid antibodies,[5] anticardiolipin antibodies in HCV patients[6] and malignancy. The most important risk factors are immunosuppressive drugs-cyclosporine,[7] tacrolimus[8] and mTOR inhibitors.[9] Other drugs implicated are antiviral agents like ganciclovir[10] and clopidogrel. Ganciclovir induced TMA is very rare with few case reports in literature. Lee et al. reported a case of TMA in a patient with autologous stem cell transplant on ganciclovir. The case in our series was a patient with existing cytomegalovirus disease who developed TMA after initiation of treatment with ganciclovir with falling viral load.
The association of cyclosporine and TMA was first described by Shulman et al.,[11] in 1981. With the introduction of tacrolimus into clinical trials in 1989, tacrolimus now is increasing used for immunosuppression in graft recipients. The incidence of tacrolimus associated TMA also is on the increase. In literature the overall incidence of tacrolimus associated TMA is 3-14%.[12] Tacrolimus was responsible for 25% of TMA in our series.
Pathogenesis of TMA can be summarized as endothelial injury and change in normal balance between thrombotic and antithrombotic factors in microvasculature. Risk of TMA is highest in first three months post-transplant. Females and elderly males are more susceptible. In our series TMA occurred from second day post-transplant to the 10th month post-transplant. Oldest patient in our series was 52 year old gentleman, there was a male predominance in our series, in contrast to what is described in literature.
Prognosis in de novo TMA is better than in recurrent disease. Prognosis is good when only glomerular lesions are seen.[13] Graft loss is rare in renal limited disease but is high in systemic disease[12] Treatment of TMA is to either lower the dose or withdrawal of offending agent. A high success rate of 84% is reported with plasma exchange therapy.[14] 5% of patients have mutations of CFH NCF1. These mutation indicate genetic complement abnormalities and represent an important risk factor.[15]
Incidence of recurrent haemolytic uremic syndrome ranged from 4% to 60% in literature. In our study incidence was 16.6%. The cause of original disease influences recurrence and is highest in familial forms and is approximately 100%.[16] The time interval between transplant and recurrence varies from few days to 2 years. 60% occur in the first month. Risk of recurrence increases with post-transplant viral infections, which activate complement.
One year graft survival is 32% for cadaveric graft and 50% for live donors.[17]
Conclusions
TMA is a morphological expression of various etiological agents in post-transplant patients. In our series, diverse etiological factors were responsible for TMA, varying from common factors like CNI toxicity to rare causes like sirolimus and ganciclovir induced disease. It is essential to be familiar with various factors because histological findings are similar, irrespective of the etiological factors.
It is of paramount importance to have a proper and complete history and correlate with other ancillary parameters to make a correct in diagnosis in a proper setting. It is important for the pathologist and the nephrologists to be aware of rare etiological causes of TMA as treatment modality varies according to the etiological factor.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant. 2003;3:1289-94.
- [Google Scholar]
- De novo hemolytic uremic syndrome postrenal transplant after cytomegalovirus infection. Am J Kidney Dis. 1999;34:556-9.
- [Google Scholar]
- Thrombotic microangiopathy associated with parvovirus B 19 infection after renal transplantation. J Am Soc Nephrol. 2000;11:1132-7.
- [Google Scholar]
- BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345:1250-5.
- [Google Scholar]
- Causes of acute thrombotic microangiopathy in patients receiving kidney transplantation. Exp Clin Transplant. 2004;2:268-72.
- [Google Scholar]
- Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol. 1999;10:146-53.
- [Google Scholar]
- Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int. 1999;55:2457-66.
- [Google Scholar]
- Tacrolimus-associated hemolytic uremic syndrome: A case analysis. J Nephrol. 2003;16:580-5.
- [Google Scholar]
- Thrombotic microangiopathy during ganciclovir treatment for cytomegalovirus infection in a patient with autologous hematopoietic stem cell transplantation. Clin Pediatr Hematol Oncol. 2011;2:161-4.
- [Google Scholar]
- Nephrotoxicity of cyclosporin A after allogeneic marrow transplantation: Glomerular thromboses and tubular injury. N Engl J Med. 1981;305:1392-5.
- [Google Scholar]
- De novo thrombotic microangiopathy in renal transplant recipients: A comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471-9.
- [Google Scholar]
- Follow-up of kidney graft recipients with cyclosporine-associated hemolytic-uremic syndrome and thrombotic microangiopathy. Transplant Proc. 2005;37:1889-91.
- [Google Scholar]
- Complement mutation-associated De novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 2008;8:1694-701.
- [Google Scholar]
- Thrombotic microangiopathy after kidney transplantation. Am J Transplant. 2010;10:1517-23.
- [Google Scholar]







