Translate this page into:
Thyrotoxic Periodic Paralysis: An Enigmatic Disease Diagnosed Incidentally Following High Dose Steroid Use
Corresponding author: Kalathil K Sureshkumar, Division of Nephrology and Hypertension, Medicine Institute, Allegheny General Hospital, Allegheny Health Network, Pittsburgh, PA, United States, Email: kalathil.sureshkumar@ahn.org
-
Received: ,
Accepted: ,
How to cite this article: Sureshkumar KK, Nashar K. Thyrotoxic Periodic Paralysis: An Enigmatic Disease Diagnosed Incidentally Following High Dose Steroid Use. Indian J Nephrol. doi: 10.25259/IJN_133_2025
Dear Editor,
Thyrotoxic periodic paralysis (TPP) is an uncommon condition. Up to 2% of East Asians and 0.1-0.2% of white Americans with hyperthyroidism can develop TPP.1,2 Stimuli increasing β-adrenergic drive or insulin levels can trigger TPP.
A 30-year-old healthy white male received intravenous high-dose methylprednisolone for allergic skin rash. The next day, he suddenly developed bilateral upper and lower extremity weakness and palpitations. He was tachycardic with flaccid paralysis and diminished tendon reflexes. An electrocardiogram revealed atrial fibrillation. Complete blood count and basic metabolic panel were unremarkable except for serum potassium of 1.4 mmol/L (3.6-5.2). The patient received intravenous and oral potassium replacement. Thyroid function tests done due to atrial fibrillation showed a low thyroid stimulating hormone level (0.007mcIU/mL; normal range: 0.4-4.0), elevated free T4 (4.28 ng/dL normal range: 0.7-1.9), and free T3 (11.19 pg/mL; normal range: 1.76-3.68) diagnostic of hyperthyroidism. He was started on propranolol and methimazole. Muscle weakness resolved completely within 12 hours of serum potassium normalization. He was stable and discharged with a planned follow-up.
TPP pathogenesis has been depicted in Figure 1. Thyroid hormones increase tissue responsiveness to β-adrenergic stimulation, increase Na-K-ATPase activity on skeletal muscle membranes, and trigger intracellular potassium shift, resulting in sarcolemmal hyperpolarization and relative inexcitability of muscle fibers. A high steroid dose likely caused hyperglycemia and insulin release by modulating thyroid hormones’ potassium-lowering effect.3 Many patients with TPP have altered expression of a gene (KCNJ2) encoding inwardly rectifying potassium channels of skeletal muscles (Kir2.6) that are transcriptionally regulated by thyroid hormones.4 Early diagnosis and prompt treatment prevent life-threatening complications like hypokalemia and muscle weakness. The diagnosis is generally delayed, on average, for 14 months.5 Acute paralysis after steroid use should raise suspicion of underlying TPP.
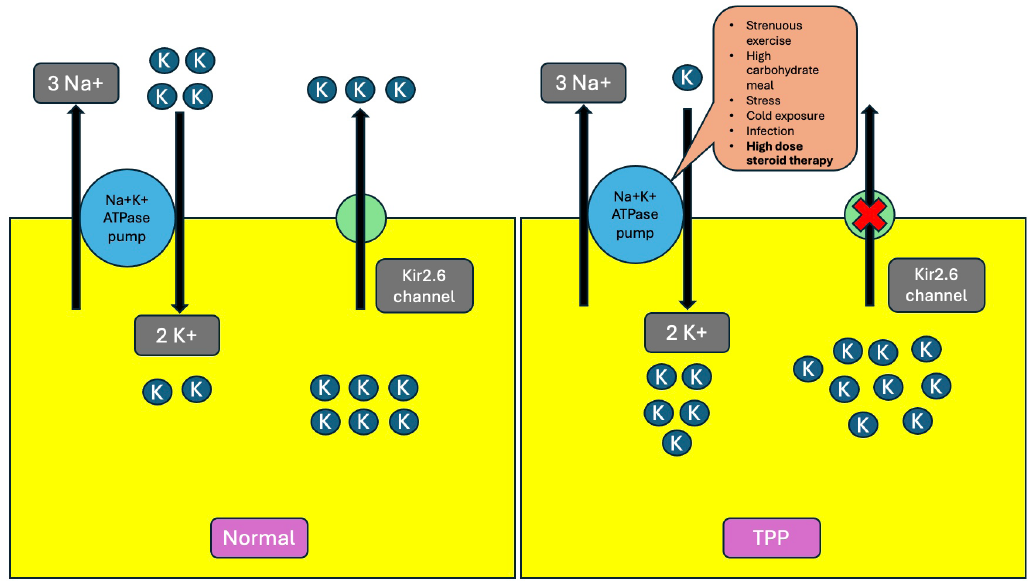
- Pathophysiology of thyrotoxic periodic paralysis. In normal skeletal muscle cells, potassium diffuses out the Kir2.6 channel and gets pumped into the cell by Na-K ATPase. In TPP, the mutated Kir2.6 channel gets dysfunctional, and Na-K ATPase gets overactive in the presence of excess thyroid hormones, resulting in massive intracellular potassium shift and severe hypokalemia following triggers. The red cross sign in the figure means the pump is blocked. Na: Sodium, K: Potassium, ATP: Adenosine triphosphate, TPP: Thyrotoxic periodic paralysis.
Conflicts of interest
There are no conflicts of interest.
References
- Thyrotoxic periodic paralysis Report of 10 cases and review of electromyographic findings. Arch. Intern. Med.. 1989;149:2597-600.
- [CrossRef] [PubMed] [Google Scholar]
- Thyrotoxic periodic paralysis in the United states Report of 7 cases and review of the literature. Medicine (Baltimore). 1992;71:109-20.
- [CrossRef] [PubMed] [Google Scholar]
- Glucocorticoids may trigger attacks in several types of periodic paralysis. Neuromuscul. Disord.. 2009;19:217-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell. 2010;140:88-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Novel etiopathologic aspects of thyrotoxic periodic paralysis. Nat Rev Endocrinol. 2011;7:657-67.
- [CrossRef] [PubMed] [Google Scholar]






