Translate this page into:
Transforming Growth Factor-Beta 1 and Endoglin Levels in Congenital Solitary Functioning Kidney
Address for correspondence: Dr. Nuran Cetın, Department of Pediatric Nephrology, Faculty of Medicine, Eskisehir Osmangazi University, TR-26480, Eskisehir, Turkey. E-mail: nurancetin17@hotmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Glomerular hyperfiltration leads to hypertension, microalbuminuria, and impaired renal function in children with congenital solitary functioning kidney (cSFK). The purpose of this study was to investigate the associations between serum transforming growth factor β-1 (TGF) and endoglin levels and hypertension, renal function or microalbuminuria in children with cSFK.
Materials and Methods:
63 patients and 36 controls were included in the study. Serum endoglin and TGF-β1 level was measured using ELISA commercial kits.
Results:
Serum TGF-β1 and endoglin levels were higher in patients than those of controls (P = 0.04 and P < 0.001, respectively). The prevalence of hypertension was found to be 45.6%. There was a positive association between endoglin levels and the presence of masked hypertension (odds ratio: 1.121, P = 0.04). TGF-β1 and endoglin levels were positively associated with microalbuminuria (OR: 1.17, P = 0.04; OR: 1.836, P = 0.01). ROC curve analysis showed that serum endoglin and TGF-β1 levels had predictive value for microalbuminuria (cut-off value: 4.86 ng/mL, sensitivity: 94.7%, specificity: 54.5%, area under the curve ± standard error [AUC ± SE]: 0.888 ± 0.025, P = 0.01 for endoglin; cut-off value 561.24 pg/mL, sensitivity: 89.5%, specificity: 73%, AUC ± SE: 0.995 ± 0.334, P = 0.02 for TGF-β1). There were no significant relationships between glomerular filtration rate and serum TGF-β1 or endoglin levels.
Conclusions:
Endoglin and TGF-β1 may play an important role in the pathophysiology of microalbuminuria in cSFK. Endoglin may have a role in the development of hypertension in children with cSFK.
Keywords
Children
congenital solitary functioning kidney
endoglin
microalbuminuria
transforming growth factor-beta 1
Introduction
Children with a solitary functioning kidney (cSFK) have a risk of renal injury caused by hyperfiltration in early life. These children are also at increased risk for hypertension, proteinuria, and renal failure in later life.[1] It has been reported that hyperfiltration-mediated injury due to the alterations in intrarenal hemodynamics can contribute to developing a renal injury. However, the exact mechanism of hyperfiltration-mediated injury remains unclear.[2] Transforming growth factor-beta 1 (TGF-β1) is a multifunctional polypeptide with a molecular weight of 25 kDa that plays a critical role in the regulation of cell proliferation, inflammation, and angiogenesis. Increased TGF-β1 expression contributes to structural changes in the artery wall and vascular fibrosis during hypertension.[3]
Endoglin is a transmembrane glycoprotein that is a part of the TGF-receptor complex and highly expressed in vascular endothelial cells in the areas of vascular injury. The serum endoglin level is thought to be a useful indicator of endothelial injury and inflammation, and endoglin levels can be increased by hypoxia and angiotensin II during renal ischemia.[4]
We aimed to investigate the serum TGF-β1 and endoglin levels in children with cSFK. We also evaluated whether serum TGF-β1 and endoglin levels were associated with hypertension, glomerular filtration rate (GFR), and urine microalbumin levels.
Materials and Methods
Study group
This study included patients aged 5–18 years with unilateral renal agenesis (URA) and multicystic dysplastic kidney (MCDK) who were followed up in our Pediatric Nephrology Outpatient Clinic between September 2010 and March 2017. Exclusion criteria were a chronic disease, active infection, congenital heart disease, vascular disease, other kidney abnormalities, and use of antihypertensive drugs at the beginning of the study.
The study includes patients’ sex- and age-matched controls. The control group's physical examination findings and office and/or ambulatory blood pressure (BP) values were normal. There was no evidence of chronic disease, active infection, or history of drug use within the past month.
Laboratory data
Peripheral venous blood samples were obtained in the morning after an overnight fast. Serum creatinine and blood urea nitrogen levels were determined on blood specimens in the patient and control groups. GFR and urine microalbumin levels were measured in the 24-h urine samples. Renal clearance of endogenous creatinine (CrCs) was calculated using the formula CrCs = UCr × V × 1.73/sCr × t × BSA (UCr = urine creatinine excretion, V = volume of urine over a given period, t = period of urine collection [24 h (1,440 min)]). Microalbuminuria was defined as excretion of 30–300 mg of albumin per 24 h. Trained personnel measured office blood pressure values by manual auscultation with a mercury sphygmomanometer.
Venous blood samples were centrifuged at 2000 g (10 min) to remove the plasma and serum. Supernatants were frozen at −80C° until further use. Serum endoglin and TGF-β1 level were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (endoglin, Elabsience-AK0017APR17031; TGF-β1, Invitrogen-145440021). As a heterogenous assay, ELISA separates some components of the analytical reaction mixture by adsorbing certain components onto a solid phase that is physically immobilized. Absorbance readings of microplates and calculations were performed using VICTOR X3 (Perkin Elmer, Waltham, United States), the results were expressed as ng/mL and pg/mL, respectively.
Ambulatory blood pressure monitoring
Ambulatory blood pressure monitoring (ABPM) was performed over 24 h using the Scanlight II/III long-term BP monitoring system. BP was measured every 20 min during the day (08:00–22:00) and every 30 min at night (22:00–08:00). Hypertension was defined as average systolic BP (SBP) and/or diastolic BP (DBP) above the 95th percentile, according to gender, age, and height. Dipping status was greater than a 10% reduction in nocturnal average SBP and/or DBP.
Ethics committee approval
This study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki. The parents of the patients and controls gave informed consent.
Statistical analysis
Statistical analyses were performed using SPSS 11.0 (SPSS Inc., Chicago, IL). Values are expressed as mean and standard deviation (SD) for continuous variables and interquartile range (IQR) for qualitative variables. The Shapiro–Wilk test was used to determine data normality. Means were compared using independent sample t-tests in normally distributed data. The comparison of the non-normally distributed data was done with the Mann-Whitney U test. Correlations between variables were evaluated using Pearson's or Spearman's tests, as appropriate. A P value <0.05 was considered significant. Categorical variables were compared via the Chi-square test. Linear regression analysis was performed to explore the relationship of serum endoglin and TGF-β1 level with GFR and urine microalbumin levels as the dependent variables. A logistic regression analysis was performed to determine the influence of serum endoglin and TGF-β1 level in the presence of microalbuminuria and hypertension. Receiver-operating characteristic (ROC) analysis was used to determine the cutoff values and the sensitivity/specificity of serum endoglin and TGF-β1 level.
Results
17 of 102 patients with cSFK who followed up in our outpatient clinic were excluded from the study because they did not meet the study criteria. Nearly half (45.6%) of the 85 patients with cSFK who met the inclusion criteria had hypertension (clinic hypertension: 22, masked hypertension: 17). Twenty-two patients receiving an antihypertensive drug at the beginning of the study were also excluded.
The remaining 63 patients (12 MCDK and 51 URA) and 36 healthy children were included in this study. The mean age of the patients was 10.9 ± 3.38 years. Serum creatinine and urine microalbumin levels were higher in the patients than in the control group (P = 0.02 and P = 0.02, respectively). The patients had significantly lower GFR compared to the control group (P = 0.02). Serum TGF-β1 and endoglin levels were higher in the patients than in the control group (P = 0.04 and P < 0.001, respectively, Table 1).
| Parameters | Patients (n=63) | Controls (n=36) | P |
|---|---|---|---|
| Gender (M/F) | 41/22 | 20/16 | 0.15 |
| Age (years) | 10.9±3.38 | 12.2±3.53 | 0.15 |
| Weight z-score | 0.61 (−0.93–1.02) | 0.68 (−0.77–0.92) | 0.12 |
| Height z-score | 0.4 (−1.12–0.84) | 0.51 (−0.95–1.04) | 0.21 |
| BUN (mg/dL) | 11.5±3.94 | 10.6±3.61 | 0.54 |
| Creatinine (mg/dL) | 0.6±0.25 | 0.5±0.14 | 0.02 |
| TGF-β1 (pg/mL) | 588.3±91.75 | 417±140.78 | 0.04 |
| Endoglin (ng/mL) | 5.7±2.37 | 2.4±1.28 | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 103.4±47.17 | 135.5±44.99 | 0.02 |
| 24-hour microalbumin (mg/day) | 9.82 (4.16–18.24) | 3.4 (1.90–18.68) | 0.02 |
| Office SBP z-score | 0.82 (−0.18–1.17) | 0.68 (−0.02–1.29) | 0.15 |
| Office DBP z-score | 0.42 (−0.03–0.91) | 0.69 (−0.14–1.35) | 0.22 |
Data were shown as mean±SE or median (interquartile range). BUN- blood urea nitrogen, SBP-systolic blood pressure, DBP-diastolic blood pressure, TGF-β; transforming growth factor-beta
The office SBP and DBP z-scores were similar in the patient and control groups (P = 0.37 and P = 0.47, respectively, Table 1). Seventeen (26.9%) of 63 patients had masked hypertension. Non-dipping status was identified in 35 (83.3%) patients. Nighttime SBP, 24-h SBP, DBP (24-h, day and night), nighttime SBP load, and daytime and nighttime DBP loads were higher in the patients than in the control group. Table 2 shows the detailed results of ABPM.
| Parameters | Patients (n=63) | Controls (n=36) | P |
|---|---|---|---|
| 24-h SBP values (mmHg) | 111.3±11.01 | 102.2±5.12 | 0.03 |
| Daytime SBP (mmHg) | 113 (101.25–116.75) | 109 (104–112.5) | 0.37 |
| Nighttime SBP (mmHg) | 112.9±9.50 | 93.5±4.95 | 0.04 |
| 24-h DBP values (mmHg) | 73.1±7.96 | 58.3±4.54 | 0.01 |
| Daytime DBP (mmHg) | 67.5 (61–74.75) | 62 (59.75–63) | 0.008 |
| Nighttime DBP (mmHg) | 69.4±9.13 | 53.3±3.97 | 0.005 |
| Daytime SBP load (%) | 0 (0–3.77) | 0 (0–0) | 0.16 |
| Nighttime SBP load (%) | 13 (9–27.5) | 7 (3–11) | 0.01 |
| Daytime DBP load (%) | 21 (11–39) | 5 (0–14) | 0.01 |
| Nighttime DBP load (%) | 21 (14–48.75) | 5 (0–9) | 0.02 |
| Non-dipping (n; %) | 35 (83.3%) | - | - |
| Masked hypertension (n; %) | 17 (26.9%) | - | - |
Data were shown as mean±SD or median (interquartile range). SBP, systolic blood pressure; DBP, diastolic blood pressure
The correlation analysis showed significant correlations between serum endoglin levels and urine microalbumin or 24-h SBP with daytime SBP loads (r = 0.475; P < 0.001, r = 0.401 and P = 0.03 or r = 0.614, P = 0.02, respectively) and a significant positive correlation between serum TGF-β1 and urine microalbumin levels (r = 0.421; P = 0.02). There were no significant correlations between GFR and serum endoglin or TGF-β1 levels.
Table 3 shows the detailed laboratory data of the patients with and without masked hypertension. Serum endoglin levels were higher in those patients with masked hypertension (5.6 ± 1.81/4.1 ± 0.65 ng/mL, P = 0.04, Figure 1a). Logistic regression analyses determined a positive association between serum endoglin levels and masked hypertension (odds ratio [OR]: 1.121, 95% confidence interval [CI] = 1.013–5.678, P = 0.04). ROC analyses showed that serum endoglin levels had diagnostic value for the presence of masked hypertension. The area under curve (AUC) was 0.730 ± 0.101 (cutoff value: 4.901 ng/mL, CI: 0.534–0.897, sensitivity: 87.5%, specificity: 54.5%, P = 0.04, Figure 1b).
| Parameters | Masked hypertension (+) (n=17) | Masked hypertension (−) (n=46) | P |
|---|---|---|---|
| Gender (M/F) | 12/5 | 29/17 | 0.21 |
| Age (years) | 11.3±2.98 | 10.9±3.05 | 0.19 |
| Weight z-score | 0.57 (−0.76–1.14) | 0.61 (−0.66–1.02) | 0.23 |
| Height z-score | 0.56 (−0.94–1.01) | 0.54 (−0.85–0.94) | 0.31 |
| BUN (mg/dL) | 11.2±3.04 | 9.5±2.61 | 0.68 |
| Creatinine (mg/dL) | 0.5±0.15 | 0.6±0.23 | 0.32 |
| TGF-β1 (pg/mL) | 584.1±80.28 | 583.5±70.56 | 0.14 |
| Endoglin (ng/mL) | 5.6±1.81 | 4.1±0.65 | 0.04 |
| Glomerular filtration rate (mL/min/1.73 m2) | 89.4±27.36 | 101.5±39.61 | 0.08 |
| 24-hour microalbumin (mg/day) | 14.76 (6.27–24.63) | 11.57 (5.16–19.91) | 0.07 |
| Office SBP z-score | 0.63 (−0.21–0.98) | 0.78 (−0.11–1.06) | 0.36 |
| Office DBP z-score | 0.44 (−0.04–0.87) | 0.56 (−0.08–1.09) | 0.21 |
Data were shown as mean±SE or median (interquartile range). TGF-β; transforming growth factor-beta
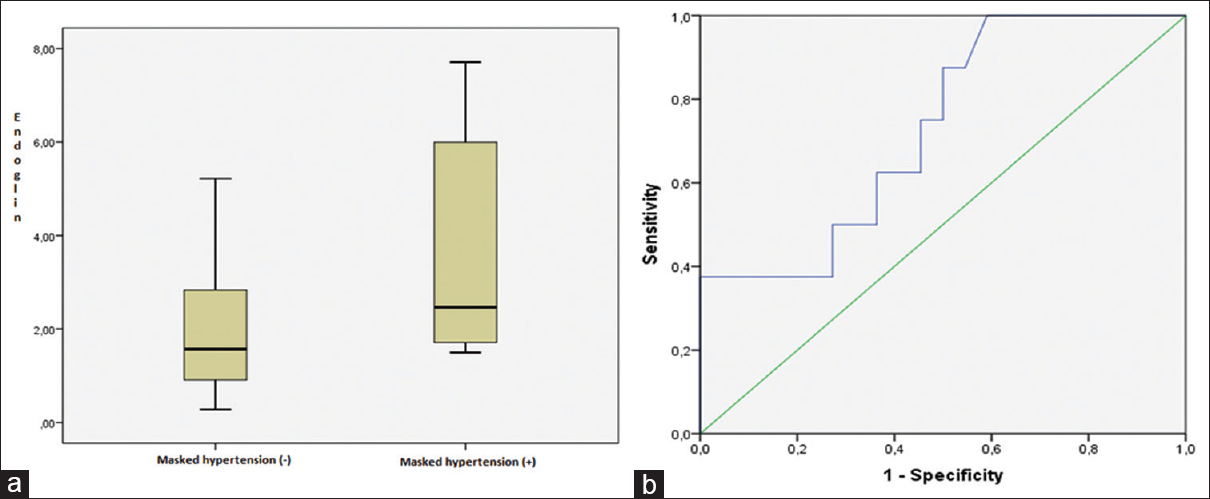
- (a) Serum endoglin levels were higher in patients with masked hypertension than those of normotensive patients (P = 0.04), (b) receiver operating characteristic (ROC) curve of serum endoglin levels for the prediction of masked hypertension. Cutoff value: 4.901 ng/mL, sensitivity: 87.5%, specificity: 54.5% (AUC ± SE: 0.730 ± 0.101, 95% CI: 0.534–0.897, P = 0.04)
Thirty-nine (61.9%) patients had microalbuminuria. Serum TGF-β1 and endoglin levels were higher in patients with microalbuminuria than in those without (P = 0.03, P = 0. 001, respectively, Table 4). A linear regression analysis determined significant associations between urine microalbumin and TGF-β1 or serum endoglin levels (beta = 0.383, P = 0.04 and beta = 0.432, P = 0.02, respectively). In logistic regression analysis, we found that serum TGF-β1 and endoglin levels had predictive value for the presence of microalbuminuria (OR: 1.17, 95% CI = 1.098–1.273, P = 0.04; OR: 1.836, 95% CI = 1.485–12.473, P = 0.01). ROC curve analysis showed that the cutoff value of endoglin for the presence of microalbuminuria was 4.86 ng/mL, with a sensitivity of 94.7% and a specificity of 54.5% (AUC ± SE: 0.888 ± 0.025, 95% CI: 0.768–1, P = 0.01, Figure 2a). TGF-β1 had a sensitivity of 89.5% and specificity of 73% with a cutoff of 561.24 pg/mL for the presence of microalbuminuria (AUC ± SE: 0.995 ± 0.334, 95% CI: 0.980–1.132, P = 0.02, Figure 2b).
| Parameters | Microalbuminuria (+) (n=39) | Microalbuminuria (−) (n=24) | P |
|---|---|---|---|
| Gender (M/F) | 25/14 | 16/8 | 0.45 |
| Age (years) | 11.4±3.41 | 9.6±2.03 | 0. 07 |
| BUN (mg/dL) | 11.5±3.96 | 11.7±4.17 | 0. 91 |
| Creatinine (mg/dL) | 0.6±0.21 | 0.6±0.18 | 0. 83 |
| TGF-β1 (pg/mL) | 598.4±54.95 | 504.4±18.96 | 0.03 |
| Endoglin (ng/mL) | 5.6±1.59 | 3.9±0.48 | 0. 001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 108.1±44.39 | 98.1±21.29 | 0. 41 |
Data were shown as mean±SE or median (interquartile range). PRA: Plasma renin activity, TGF-β: Transforming growth factor-beta

- ROC curve of serum endoglin and transforming growth factor β1 (TGF-β1) levels for the prediction of microalbuminuria in patients with congenital solitary functioning kidney (cSFK). (a) Cutoff value: 4.86 ng/mL, sensitivity: %94.7, specificity: 54.5% (AUC ± SE: 0.888 ± 0.025, 95% CI: 0.768–1, P = 0.01) for endoglin. (b) Cutoff value: 561.24 pg/mL, sensitivity: 89.5%, specificity: 73% (AUC ± SE: 0.995 ± 0.334, 95% CI: 0.980–1.132, P = 0.02) for TGF-β1
Discussion
We investigated whether serum TGF-β1 and endoglin levels were associated with microalbuminuria, hypertension, or GFR. The results of this study revealed higher ABPM values, serum TGF-β1, endoglin, and urine microalbumin levels in children with cSFK. Serum endoglin and TGF-β1 level were positively associated with microalbuminuria. However, there were no significant relationships between GFR and serum TGF-β1 or endoglin levels.
Microalbuminuria could reflect both endothelial cell dysfunction and early renal injury. The increased albumin filtration because of glomerular injury may contribute to the development of microalbuminuria by exceeding the albumin reabsorption capacity of the tubule. However, there are conflicting results for the association between microalbuminuria and glomerular hyperfiltration or tubular damage in children with cSFK.[56] We could not identify a significant association between urine microalbumin levels and GFR in patients with cSFK. Our results suggest that higher urine microalbumin excretion may be due to changes in tubular reabsorption of albumin in children with cSFK. Factors besides hyperfiltration may contribute to the development of microalbuminuria in these children.
Hypertension and microalbuminuria are early signs of renal injury in children with cSFK.[7] The structural and functional changes in glomeruli due to hyperfiltration lead to increased glomerular pressure, glomerulosclerosis, and systemic hypertension. Tabel et al. found that the mean SBP and 24-h DBP loads were higher in children with SFK than in a control group.[8] Dursun et al. showed that mean 24-h DBP values were higher in a URA group than in a control group.[9] We found that about one-third of patients had masked hypertension. However, office SBP and DBP values did not differ between the patients and the control group, suggesting that it might be worthwhile to consider ABPM in all children with cSFK.
TGF-β1 is an important mediator in the development of renal fibrosis. Fluid shear stress and the renin-angiotensin-aldosterone system in SFK activate TGF-β1 production in endothelial cells and contribute to the pathogenesis of hypertensive injury. The increased secretion of TGF-β1 causes podocyte apoptosis, decreased matrix degradation, epithelial-mesenchymal transition, and decreased mRNA levels of megalin in the kidney.[10] It has also been reported that TGF-β1 induces proteinuria by changes in the glomerular filtration barrier, such as decreased mRNA levels of nephrin and interstitial fibrosis or mesangial expansion.[111213] Suthanthiran et al. reported a positive association between serum TGF-β1 level and microalbuminuria or chronic kidney disease progression.[14] Thus, it has been suggested that anti-TGF-β therapy can decrease renal fibrosis.[1516] Miyajima et al. showed that inhibiting TGF-β gene expression could decrease interstitial fibrosis in a unilateral ureteral obstruction model.[17] We showed significant associations between serum TGF-β1 level and microalbuminuria. However, there was no significant association between GFR and serum TGF-β1 level. Our results suggest that increased TGF-β1 level may cause microalbuminuria by affecting proximal tubule cells and/or podocyte independence from GFR in children with cSFK. Future research is necessary to determine whether TGF-β1 plays a role in the development of microalbuminuria in cSFK.
The literature has conflicting results on associations between TGF-β1 and hypertension. Suthanthiran et al. showed higher serum TGF-β1 level in Caucasian hypertensive patients.[18] Laviades et al. reported that hypertensive patients with microalbuminuria had higher serum TGF-β1 level.[19] In contrast, Matsuki et al. suggested that TGF-β1 suppressed renal tubular sodium reabsorption in a mouse model and reported that elevated TGF-β1 level did not lead to hypertension.[20] We could not find significant correlations between TGF-β1 level and BP values, standing PRA, or aldosterone levels, and we could not demonstrate significant effects of serum TGF-β1 level on hypertension. Therefore, further detailed studies are needed to confirm the role of serum TGFβ1 level in BP regulation in cSFK.
Endoglin modulates the interaction between TGF and its receptors. It was thought that endoglin could be an inhibitor for TGF-β1 signaling. It has been reported that endoglin levels increased in kidneys with renal fibrosis by renal mass reduction and unilateral ureteropelvic obstruction.[2122] An animal study revealed that endoglin could reduce inflammatory infiltration in kidneys after ischemic-reperfusion injury in transgenic mice.[23] Thus, it has been suggested that increased endoglin expression could be a protective mechanism against the fibrogenic effects of TGF-β.[24] Charytan et al. investigated whether serum endoglin levels could contribute to the progression of chronic kidney disease in adult patients. They could not determine significant associations between serum endoglin levels and GFR or urine microalbumin levels.[25] We also could not identify a significant association between serum endoglin levels and GFR. However, our results revealed higher serum endoglin levels in patients with cSFK. Further, serum endoglin levels were significantly associated with microalbuminuria. This result might support endoglin as a cause of microalbuminuria in patients with cSFK.
The expression of endoglin in vascular endothelial and smooth muscle cells increases by vascular injury in endothelial cells.[22] The release of endoglin leads to decreased prostacyclin and nitric oxide production, endothelial cell dysfunction, and hypertension.[26] An adult study showed a significant correlation between serum endoglin levels and elevated BP in hypertensive and diabetic patients.[27] Our results showed that serum endoglin levels were associated with hypertension and therefore suggest that endoglin might play a role in the development of hypertension in children with cSFK.
Our study has certain limitations. It is cross-sectional and has a small sample size. We did not assess the middle- and long-term effects of endoglin and TGF-β1. The device for ABPM was not a monitor validated in children by the American Academy of Pediatrics Clinical Practice Guideline for Screening and Management of High BP in Children and Adolescents.[28] However, ABPM was performed via a standardized approach with pediatric normative data.
It has been reported that urinary TGF-β1 may serve as a marker of early renal injury in obstructive uropathies and reflux nephropathy and that urinary TGF-β1 correlates with the degree of renal interstitial fibrosis.[2930] The lack of measurement of urinary TGF-β1 and endoglin levels is also an important limitation in this study, since these levels may reflect intrarenal production. In the literature, there are few studies investigating urinary endoglin levels.[31] It was shown that patients with severe preeclampsia had increased urinary endoglin levels and that urinary endoglin levels were positively correlated with proteinuria.[32] To the best of our knowledge, there are no published reports evaluating urine endoglin levels in kidney diseases, although several clinical and experimental studies have shown increased levels of endoglin in serum, plasma, or tissue in fibrotic-related pathologies.[33] We were not able to show that urinary TGF-β1 and endoglin levels might also be associated with renal damage in children with cSFK. Nevertheless, this study was the first to test for serum endoglin and TGF-β1 level in children with cSFK.
In conclusion, the results of our study show that children with cSFK have higher urine microalbumin levels and BP values with lower GFR. The increased serum TGF-β1 level may contribute to developing microalbuminuria independent from GFR in cSFK. Serum endoglin levels may play an important role in the pathophysiology of microalbuminuria and hypertension. Long-term follow-up studies in larger groups are needed to determine the association between renal function and serum TGF-β1 or endoglin levels in children with cSFK. The results of our study provide a basis for future studies or treatment strategies to slow the progression of chronic kidney disease in cSFK.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Risk factors for renal injury in children with a solitary functioning kidney. Pediatrics. 2013;131:478-85.
- [Google Scholar]
- Nephron deficiency and predisposition to renal ınjury in a novel one-kidney genetic model. J Am Soc Nephrol. 2015;26:1634-46.
- [Google Scholar]
- Angiotensin II via activation of type 1 receptor upregulates expression of endoglin in human coronary artery endothelial cells. Hypertension. 2001;38:1062-7.
- [Google Scholar]
- Kidney function in adults born with unilateral renal agenesis or nephrectomized in childhood. Pediatr Nephrol. 1988;2:177-82.
- [Google Scholar]
- Solitary kidney. Study of renal morphology and function in 95 children. Nefrologia. 2006;26:56-63.
- [Google Scholar]
- Evaluation of hypertension by ambulatory blood pressure monitoring in children with solitary kidney. Blood Press. 2015;24:119-23.
- [Google Scholar]
- Ambulatory blood pressure monitoring and renal functions in children with a solitary kidney. Pediatr Nephrol. 2007;22:559-64.
- [Google Scholar]
- Transforming growth factor-beta1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol. 2003;552:471-81.
- [Google Scholar]
- Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest. 1996;74:991-1003.
- [Google Scholar]
- The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am J Physiol Renal Physiol. 2009;297:85-94.
- [Google Scholar]
- Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632-43.
- [Google Scholar]
- Circulating transforming growth factor- β1 levels and the risk for kidney disease in African Americans. Kidney Int. 2009;76:72-80.
- [Google Scholar]
- Oral administration of GW788388, an inhibitor of TGF- beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73:705-15.
- [Google Scholar]
- Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301-13.
- [Google Scholar]
- Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci USA. 2000;97:3479-84.
- [Google Scholar]
- Transforming growth factor beta in hypertensives with cardiorenal damage. Hypertension. 2000;36:517-22.
- [Google Scholar]
- The role of transforming growth factor β1 in the regulation of blood pressure. Curr Hypertens Rev. 2014;10:223-38.
- [Google Scholar]
- Up-regulation of endoglin, a TGF-beta-binding protein, in rats with experimental renal fibrosis induced by renal mass reduction. Nephrol Dial Transplant. 2001;16:34-9.
- [Google Scholar]
- Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between Sp1 and KLF6: Their potential role in the response to vascular injury. Blood. 2002;100:4001-10.
- [Google Scholar]
- Endoglin upregulation during experimental renal interstitial fibrosis in mice. Hypertension. 2002;40:713-20.
- [Google Scholar]
- Circulating soluble endoglin modifies the inflammatory response in mice. PLoS One. 2017;12:e0188204.
- [Google Scholar]
- Circulating endoglin concentration ıs not elevated in chronic kidney disease. PLoS One. 2011;6:23718.
- [Google Scholar]
- Endoglin regulates cyclooxygenase-2 expression and activity. Circ Res. 2006;99:248-56.
- [Google Scholar]
- Increased plasma soluble endoglin levels as an indicator of cardiovascular alterations in hypertensive and diabetic patients. BMC Med. 2010;8:86.
- [Google Scholar]
- Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:20171904.
- [Google Scholar]
- Interactions between cytokines, congenital anomalies of kidney and urinary tract and chronic kidney disease? Clin Dev Immunol. 2013;2013:597920. doi: 10.1155/2013/
- [Google Scholar]
- Urinary endothelin-1excretion according to morpho-functional damage lateralization in reflux nephropathy. Nephrol Dial Transplant. 2004;19:1774-8.
- [Google Scholar]
- Endoglin (CD105) as a urinary and serum marker of prostate cancer. Int J Cancer. 2009;124:664-9.
- [Google Scholar]
- The role of urinary soluble endoglin in the diagnosis of pre-eclampsia: Comparison with soluble fms-like tyrosine kinase 1 to placental growth factor ratio. BJOG. 2010;117:321-30.
- [Google Scholar]







