Translate this page into:
Type I Cryoglobulinemic Nephritis in a Patient of Monoclonal Gammopathy of Renal Significance
Address for correspondence: Dr. V. A. Lobo, Department of Nephrology, King Edward Memorial Hospital, 489, Rasta Peth, Sardar Moodliar Road, Pune - 411 011, Maharashtra, India. E-mail: valentinelobo@rediffmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Monoclonal gammopathy of renal significance is a recently described entity in which a small B-cell clone not meeting the criteria for the diagnosis of multiple myeloma produces renal disease usually through deposition of a secreted monoclonal immunoglobulin. Here, we describe a case of Type I cryoglobulinemic glomerulonephritis diagnosed on a kidney biopsy and caused by a monoclonal IgM produced by a small bone marrow clone. The patient made a complete renal recovery after chemotherapy to suppress the clone.
Keywords
Cryoglobulinemia
light chain restriction
monoclonal gammopathy
Introduction
Monoclonal gammopathy of renal significance (MGRS) is defined by the causal relationship between a small B-cell clone and renal disease, usually through deposition of the secreted monoclonal immunoglobulin (MIg) or a fragment thereof.[1] The spectrum of MGRS is evolving, with the recent description of novel entities.
Case Report
A 52-year-old male patient was admitted on August 1, 2013, with a history of breathlessness, lower limb swelling for 4 days, and an erythematous rash over the abdomen for 2 days. His medical history was unremarkable with no history of arthralgias or other systemic illness. He had been referred to our unit for an elevated plasma creatinine (2.69 mg%). Examination revealed a papular nonblanching erythematous rash on the thorax, abdomen, upper and lower limbs, eyelid puffiness, and bilateral pedal edema 4+. His blood pressure was elevated at 180/100 mmHg, chest auscultation revealed bilateral rales, and other systemic examination was unremarkable. Urinalysis showed 2+ albumin, 5–6 leukocytes, and 5–6 red cells per high power field, while ultrasonography showed normal sized kidneys with swollen cortex. The rest of his investigations are shown in Table 1. A skin biopsy of the papules showed hyperkeratosis, keratin plugging, and a mild mononuclear dermal infiltrate with fragmented collagen and elastin fibers. As his creatinine rose to 4.8 mg% on the 4th of August, a left renal biopsy was performed. The biopsy contained 14 glomeruli, 12 of which were enlarged and showed increased mesangial matrix, hypercellularity, focal areas of endocapillary hypercellularity, and infiltrating polymorphs. Intracapillary, periodic acid-Schiff-positive hyaline pseudothrombi were seen in 9 of the 14 glomeruli, which were fuchsinophilic on Masson's trichrome stain. Jones silver stain, revealed double contouring of the basement membrane with interposed eosinophilic material – tram track appearance. Widespread flattened tubular epithelium with loss of brush border and a diffuse mononuclear infiltrate in the interstitium were seen [Figures 1–4]. Immunofluorescence showed 2+ diffuse coarse granular deposits of IgM and kappa light chains. As the above findings suggested a cryoglobulinemic glomerulonephritis with monoclonal light chains, the serum-free light chains, protein electrophoresis, and cryoglobulins were determined as shown in Table 1 and Figure 5a. A bone marrow aspiration and biopsy revealed only 3% plasma cells. In view of the excess free light chains with kappa restriction in the serum and the kidney, monoclonal cryoglobulins, acute kidney injury secondary to cryoglobulins, and a plasma cell clone not fitting the diagnosis of multiple myeloma, a diagnosis of MGRS was made. The patient was treated with an intravenous dexamethasone 40 mg daily for 5 days every month for 4 months, followed by oral cyclophosphamide 250 mg fortnightly and dexamethasone 20 mg weekly till December 2014 and 100 mg of oral thalidomide daily. His serum creatinine decreased to 1.54 mg% in December 2013 and decreased to 1.13 mg% in December 2014. In December 2014, a repeat serum protein electrophoresis study showed a disappearance of the monoclonal b and [Figure 5b] and a reduction in the kappa light chains to 4.71 ng/ml with a normal ratio of 1.8:1. The rest of his reports are shown in Table 2. The patient continues to be in complete hematological and renal remission and is being monitored for clone activity every 6 months.

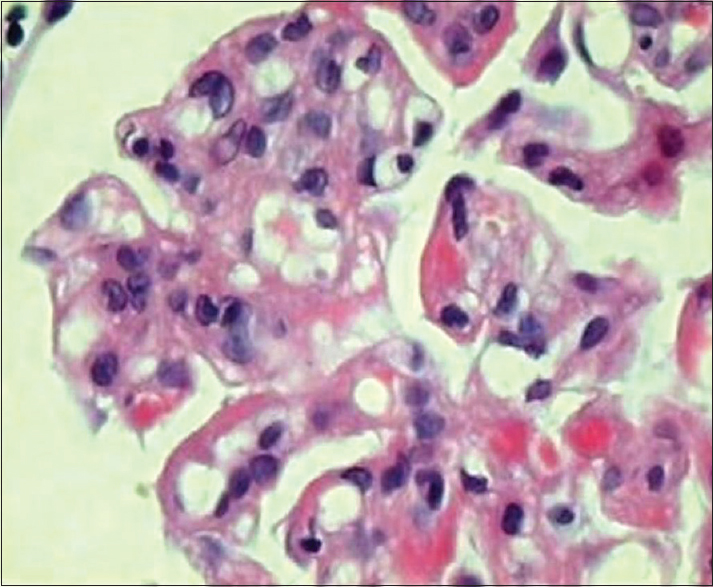
- Glomerulus showing increased cellularity, eosinophilic deposits, and thickened basement membrane (H and E, ×450)
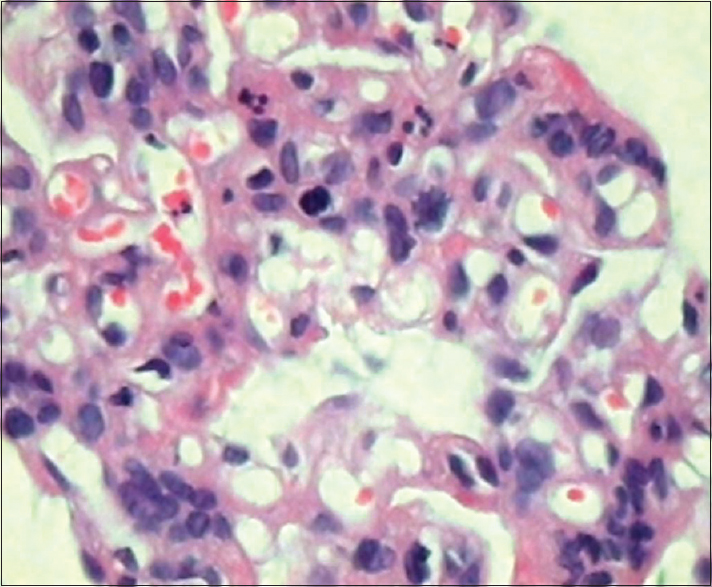
- Marked increase in mesangial cells and a few polymorphonuclear leukocytes (H and E, ×450)

- Intracapillary homogeneous eosinophilic pseudothrombi composed of cryoglobulins (H and E, ×450)
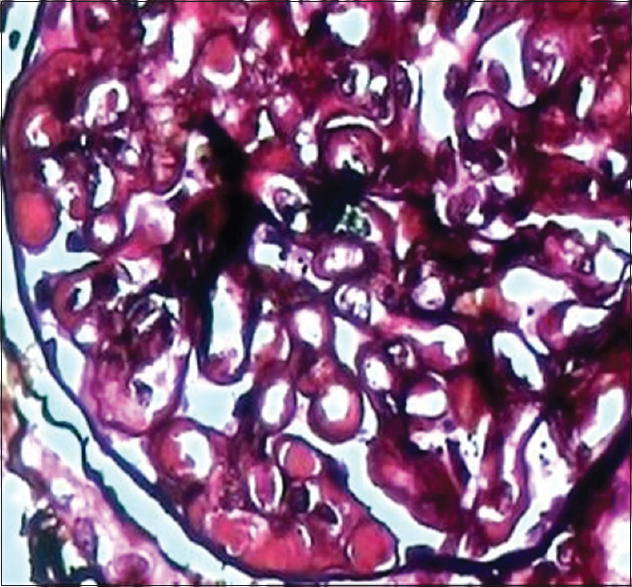
- Cryoglobulin pseudothrombi and double contouring of basement membrane (Jones silver, ×450)
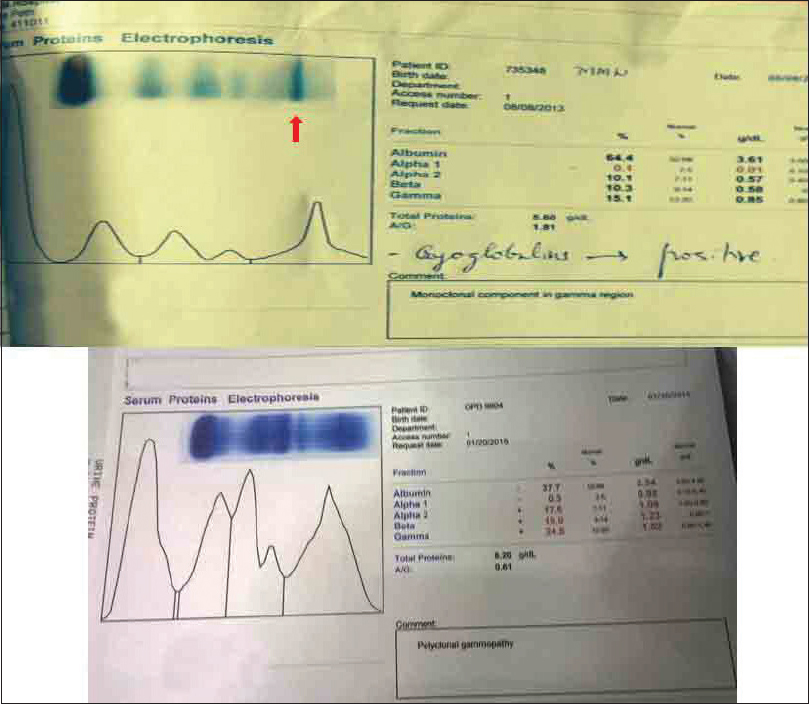
- Protein electrophoresis. A sharp monoclonal band is seen (arrow), which has been replaced with a polyclonal pattern after 14 months of treatment

Discussion
Historically, plasma cell disorders have been classified as monoclonal gammopathy of unknown significance (MGUS), smoldering myeloma, indolent myeloma, and multiple myeloma.[2] While MGUS is present in around 3.5% of the population above 50 years, traditionally, it has a low risk of progressing (around 1%/year) to multiple myeloma, other lymphoproliferative disorders or primary amyloidosis.[2] MGRS is a recently emerging entity defined as a causal relationship between a small B-cell clone and renal damage usually through the deposition of the secreted MIg, or indirectly through dysregulation of the alternative pathway of complement, and not directly related to cellular proliferation.[34] A clear distinction is made from MGUS without any end organ damage to a condition with severe consequences of MIg deposition in the kidneys and extrarenal organs, that considerably increases morbidity, impairs patient survival, and in the absence of efficient suppression of the cell clone recurs in an allograft.[5]
Sethi et al.[6] at the Mayo Clinic in a retrospective analysis reported 28 cases with a membranoproliferative glomerulonephritis (MPGN) pattern of injury caused by intact MIgs. Sixteen of these were classified as MGUS, however, in view of the significant renal injury, proteinuria, and altered renal function the authors proposed the name “Monoclonal gammopathy associated MPGN.” Six of the patients in their series had cryoglobulinemic features on light microscopy of the renal biopsy. Nasr et al.[7] have reported a case of Type I cryoglobulinemia with MPGN in a 57-year-old patient who also had arthritis and a positive ANA and a late appearance of Type I cryoglobulin composed of IgG kappa in the serum 2 months later. They also reported that until 2006 only 21 cases of glomerulonephritis secondary to Type I cryoglobulins were reported in the English literature. Although 16 of the patients had elevated serum creatinine, only 8 had hematological malignancies. The predominant histology in their case and the other 21 was MPGN as in our patient, while 7 patients in their review had intracapillary pseudothrombi as seen in our patient. While they report IgG kappa as the most common circulating immunoglobulin, our patient was found to have a monoclonal IgM kappa both on protein electrophoresis and on immunofluorescence studies on the renal biopsy, which became undetectable in the serum after 1 year of treatment. In the patient described by them also the bone marrow contained only 5% atypical plasma cells, but the patient was treated with low-dose dexamethasone and thalidomide. In our case, as in the series described by Sethi et al., it was the renal biopsy that dictated the subsequent evaluation for a monoclonal gammopathy with electrophoresis studies, free light-chain assays, and a bone marrow biopsy. Kidney biopsy is always indicated in a patient with clinical and laboratory features consistent with MGRS, and its careful assessment may even reveal MGRS in the absence of a detectable light chain clone by serum urine or bone marrow testing.[5]
Traditionally, treatment has not been recommended for any of these conditions except multiple myeloma, while the others are monitored until progression occurs. This means that only 65% of patients with monoclonal immunoglobulin deposition disease and 30% of AL amyloidosis receive appropriate treatment.[8] This approach may have been driven by the understanding that the overall survival of MGRS is better than that of multiple myeloma and AL amyloidosis, but unfortunately, the renal outcomes are not and a high rate of recurrence after transplantation if the original clone persists has been found. Our patient had an IgM kappa light chain cryoglobulinemia, which has previously been reported in Waldenstrom's macroglobulinemia,[910] and successfully treated with prednisolone and cyclophosphamide on one occasion and with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone on the other. As no treatment currently arrests the deposition of immunoglobulins in the kidney, targeting the B-cell clone, although not an evidently malignant one with chemotherapy, adjusted for renal and extrarenal toxicity has been suggested. In our patient, we used cyclophosphamide for which widespread experience in renal failure exists and thalidomide which is nonrenally cleared. We also offered bortezomib therapy, which our patient refused and obtained a complete hematological and renal remission, which continued till the last follow-up 2½ years after the original diagnosis.
Conclusions
Our case illustrates that the diagnosis and subsequent investigations of MGRS conditions may be indicated by the renal biopsy findings and that therapy targeting a small B-cell clone even in the absence of definite evidence of criteria for hematological malignancy may be associated with excellent recovery and prognosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors gratefully thank Dr. S. S. Naik of the Department of Biochemistry for his excellent support.
References
- Monoclonal gammopathy of renal significance: When MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292-5.
- [Google Scholar]
- A history of the kidney in plasma cell disorders. In: Herrera GA, ed. The kidney in plasma cell dyscrasias. Contrib Nephrol. Vol 153. 2007. p. :5-24.
- [Google Scholar]
- Monoclonal gammopathy of renal significance: Why is it significant? J Blood Disord. 2014;1:2-3.
- [Google Scholar]
- The use of immunoglobulin light chain assays in the diagnosis of paraprotein-related kidney disease. Kidney Int. 2015;87:692-7.
- [Google Scholar]
- C3 glomerulonephritis associated with monoclonal gammopathy: A case series. Am J Kidney Dis. 2013;62:506-14.
- [Google Scholar]
- Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol. 2010;5:770-82.
- [Google Scholar]
- Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698-711.
- [Google Scholar]
- Membranoproliferative glomerulonephritis complicating Waldenström's macroglobulinemia. BMC Nephrol. 2012;13:172.
- [Google Scholar]
- Renal lesions associated with IgM-secreting monoclonal proliferations: Revisiting the disease spectrum. Clin J Am Soc Nephrol. 2008;3:1339-49.
- [Google Scholar]







