Translate this page into:
Uncracking a Case
Address for correspondence: Dr. Filipa S Cardoso, Centro Hospitalar Lisboa Central EPE, Hospital Curry Cabral, Nephrology, R. Beneficência 8, 1050-099 Lisboa, Portugal. E-mail: cardoso.fsantos@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Nutcracker phenomenon (NCP) refers to compression of the left renal vein (LRV), most frequently between the aorta and the superior mesenteric artery, with impaired blood outflow often accompanied by distention of the distal portion of the vein. The nutcracker syndrome (NCS) is the terminology used when the nutcracker phenomenon is accompanied by a complex of symptoms such as pain (abdominal, flank, and pelvic), hematuria and orthostatic proteinuria. Diagnosis can be made with Doppler ultrasound (DUS), computed tomography (CT), magnetic resonance imaging (MRI), and venography. We describe a case of a young female adult who was identified with NCP by DUS, after a first CT had found no abnormalities. She presented with flank pain and severe hematuria causing a drastic decrease in hemoglobin levels. The management of NCS depends upon the clinical presentation and the severity of the LRV hypertension. The treatment options range from conservative to nephrectomy. Treatment decision should be based on the age of patients, severity of symptoms, and their expected reversibility. This case describes an underreported disorder that presents with non-specific symptoms, demonstrating the difficulties of the diagnostic approach of NCS, as well as the challenges in the appropriate management, given the lack of standardized treatment.
Keywords
Hematuria
left renal vein
nutcracker syndrome
Introduction
Nutcracker phenomenon (NCP) was first defined anatomically by Grant in 1937,[1] as the compression of the left renal vein (LRV) between the aorta and the superior mesenteric artery, resembling a nut between the jaws of a nutcracker. This leads to stenosis of the aortomesenteric portion of LRV and dilation of the distal portion of the vessel.[2] Nutcracker syndrome (NCS) is the terminology used when NCP is accompanied by signs and symptoms, such as hematuria, abdominal pain, and orthostatic proteinuria. This term is often used to describe both NCS and NCP, but the latter refers only to the anatomical abnormality.[3]
NCS's exact prevalence is unknown, mainly due to the variability of symptoms and absence of diagnostic criteria, but appears to be higher in females. Patients' age can range from childhood to the seventh decade but is more prevalent in young (second or third decade of life) and middle aged adults.[2] Several imaging methods such as Doppler ultrasonography (DUS) or computed tomography (CT) are used to diagnose NCS. Treatment options range from conservative to surgery, depending on clinical presentation and severity of LRV hypertension.[4]
Case Report
We present a case of a 28-year-old female, with no medical history or known medication. She presented to the emergency room (ER) with macroscopic hematuria and bilateral back pain that started 2 days ago. Physical examination and vital signs were normal. Gynecological disorders were excluded. Blood samples showed hemoglobin (Hb) of 134 g/L, normal kidney function (serum urea 4.5 mmol/L and serum creatinine 58 μmol/L), 24-hour proteinuria of 1.5 g/L, and urinanalysis with hematuria of 11266/uL, with mostly eumorphic erythrocytes. The remaining blood tests were unremarkable. Renal ultrasound showed normal-sized kidneys with normal corticomedullary differentiation. In order to rule out an urologic disease, a cystoscopy, an abdominal computed tomography (CT) with contrast and a CT urogram were performed, that showed no abnormalities. Because no diagnosis was made, she was discharged and started follow-up in nephrology clinic. Despite remaining asymptomatic, macroscopic hematuria persisted for 4 months. Complement levels, antinuclear antibody, antidouble stranded DNA (anti-dsDNA), serologies for hepatitis B, C, and human immunodeficiency virus and serum protein electrophoresis were negative. Five months later she presented with de novo ferropenic anemia (Hb 92 g/L, iron 2.9 μmol/L, ferritin 2.3 μg/L and transferrin saturation 3.5%), even though she had a spontaneous improvement on hematoproteinuria (161/μL and 0.18 g/L), which improved with both intravenous and oral iron supplementation. A kidney biopsy was proposed, but the patient refused. The flank pain persisted sporadically, mainly unilateral, strong enough that the patient had to go to the ER several times. A high level of suspicion for NCS prompted further investigation with DUS that showed stenosis of the aortomesenteric portion of LRV and dilation of its' distal portion [Figure 1a]. Measurement of the stenosis revealed a LRV diameter change from 11.1 mm [Figure 1b] to 1.8 mm [Figure 1c], with an estimated ratio of 6.2. Velocity measurements in the hilar segment of LRV were 19 cm/s [Figure 2a]. At the mesoaortic portion, the velocity measurement was 135 cm/s. This produces a velocity ratio of 7.1 [Figure 2b]. These abnormalities were consistent with NCS. Afterward, CT images were reviewed, showing a stenosis of LRV by superior mesenteric artery (SMA) [Figure 3]. We contacted vascular surgery to decide the best course of action. Even though she had occasional flank pain and her hematuria was so pronounced that she became anemic, a year had passed since her hemoglobin levels dropped and she was otherwise stable. As such, we decided on a conservative approach.
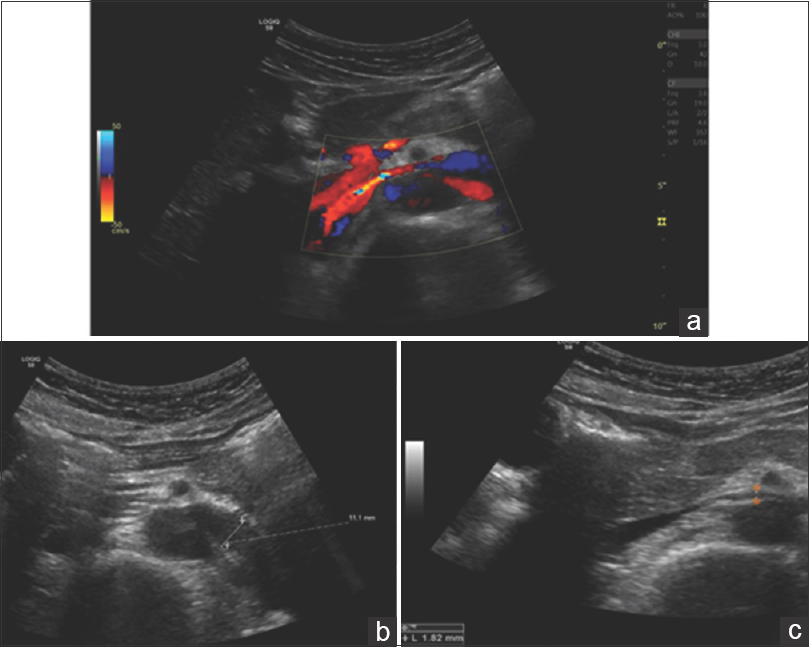
- Doppler ultrasound, transversal scan. (a) Color Doppler image of LRV showing a reduction in anteroposterior (AP) diameter of mesoartic segment and “aliasing” indicating a turbulent flow and an increase in velocity. LRV AP diameter ratio: (b) Hilar segment has an estimated AP diameter of 11.1mm (c) Mesoaortic segment with an estimated AP diameter of 1.8 mm; hilar AP diameter/mesoaortic AP diameter 6.2
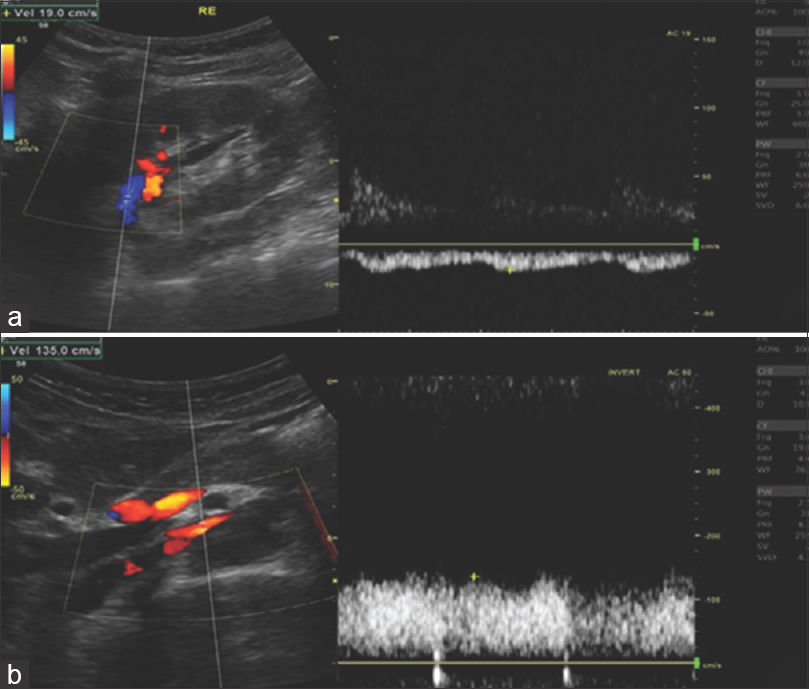
- Doppler ultrasound. Peak systolic velocity ratios between hilar and mesoaortic segments of LRV. (a) Peak systolic velocity of hilar segment estimated in 19 cm/s; (b) peak systolic velocity of mesoaortic segment estimated in 135 cm/s. Ratio 7.1
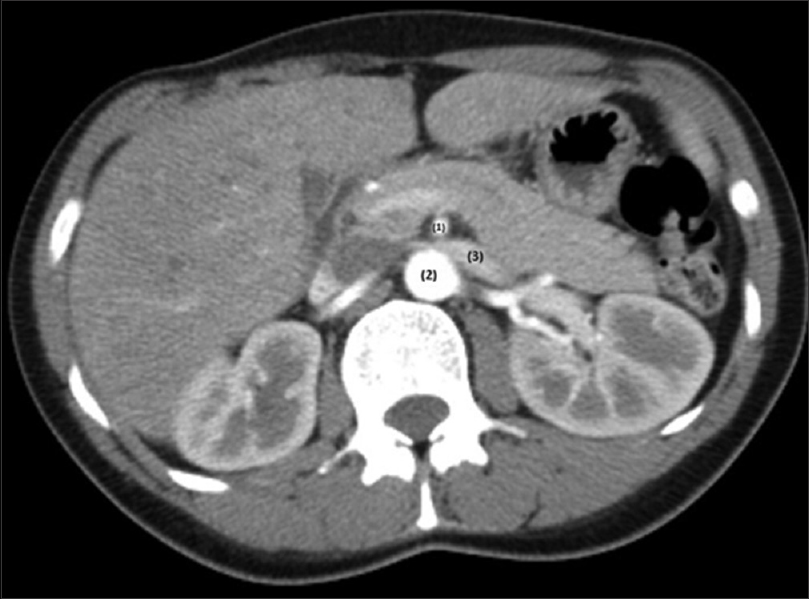
- Computer tomography; axial plane. (1) SMA; (2) aorta; (3) LRV; there is a left renal vein compression between aorta and superior mesenteric
Discussion
The patient above demonstrates a case of NCS. The exact prevalence of NCS is unknown. It can be found at any time from childhood to old age, with higher prevalence in young- and middle-aged adults. During puberty, a rapid increase in body height leads to narrowing of the angle between the SMA and the abdominal aorta, predisposing to NCP. Furthermore, a low body mass index correlates positively with NCS, given the less amount of fat and fibrous tissue surrounding the LRV.[5]
NCS refers to the extrinsic narrowing of the LRV between the abdominal aorta and the proximal SMA accompanied by specific symptoms. These symptoms are related to the compression of the LRV and consequent kidney venous congestion and increased resistance against arterial influx. The symptoms associated with NCS are grouped into two categories: the renal symptoms that include hematuria, orthostatic proteinuria, and flank pain; and the urologic symptoms, composed by abdominal pain, varicocele, dyspareunia, dysmenorrhea, fatigue, and orthostatic intolerance.[4] In the presented case, the patient manifested with hematuria and flank pain. Even though hematuria is the most common clinical feature and often what drives a patient to seek medical attention, with just these two symptoms, the source of her problem may not be clear at first sight, requiring further diagnostic investigation.
Several methods are used to diagnose NCS, including DUS, CT angiography, MRI, and venography. DUS is the recommended initial diagnostic modality, with a sensitivity and specificity as high as 78% and 100% respectively. It can identify tortuous pelvic vessels with large diameters (5-6 cm) and decreased or reversed flow velocities. Radiologic criteria include: peak systolic velocity and diameter ratios between mesoaortic and hilar segments of the LRV greater than 5; distance between posterior wall of SMA and anterior wall of the aorta under normal limits [5-6 mm; Figure 4a] and an acute angle origin of the SMA from the aorta (normal values reported 54 ± 5 degrees; Figure 4b] – all of which our patient presented. Retrograde venography is considered the gold standard for the diagnosis of NCS; however, it is an invasive diagnostic approach and should be used only when the other methods are unable to provide a diagnosis. Other modalities used for the diagnosis of NCP include CT and MRI, which demonstrate a tighter angle between the LRV and the superior mesenteric artery, often less than 35°. They can clearly depict the LRV with related structures and organs, but the measurement of flow velocity and direction are insufficient.[6]
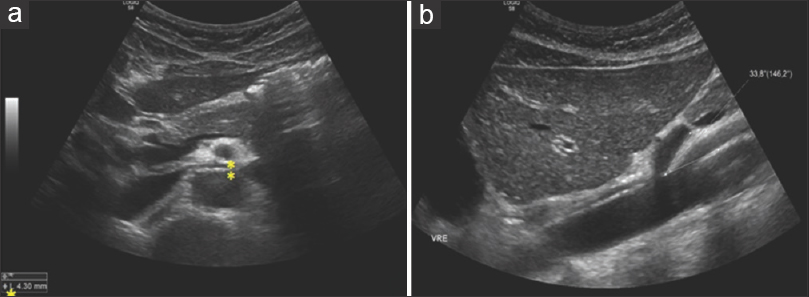
- (a) Transversal scan. Distance between SMA (posterior wall) and aorta (anterior wall) measure approximately 4 mm (normal values are 5-6 mm). (b) Longitudinal scan. Acute angle origin of the SMA from the aorta (normal values reported as 54 degrees +/- 5 degrees
The patient described above underwent a CT, which, in theory, would diagnose the NCS; however, this went unnoticed, which is not unusual, given the variations in normal anatomy and the lack of awareness of professionals for this uncommon condition. Moreover, it is also important to remember that accurate examinations require the patient to be in both upright and supine positions, in order to increase the diagnostic sensitivity. Persistent symptoms and a high level of suspicion prompted further investigation with Doppler ultrasonography, allowing a final diagnosis of NCS to be made.
Treatment of NCS varies based on the age of the patient and the scope of symptoms. Patients with less than 18 years of age are managed conservatively, with many patients experiencing spontaneous resolution of the anatomical abnormality and symptoms with physical development and growth. Likewise, increasing the body mass index can also lead to remission of symptoms, because the increased fat and fibrous tissue will relieve the compression on the LRV. In regards of pharmacological therapy, angiotensine-converting enzyme inhibitors seem to improve orthostatic proteinuria, aspirin appears to improve left to right renal perfusion ratios and analgesics may be used for pain control.[5]
It is valid to maintain a conservative approach for roughly 24 months in the case of patients with less than 18 years of age and 6 months for older patients. After this time, if the patient remains persistently symptomatic, surgery is the recommended course of care. Among the various surgical modalities, endovascular stenting of the renal vein is the initial therapy of choice. If endovascular surgery is not successful, other surgical procedures such as nephropexy, transposition of the LRV or superior mesenteric artery, renal autotransplantation, or even nephrectomy have been implemented as treatment.[5]
In the case described above, the initial approach was conservative. Since the patient remained symptomatic and even had a considerable decrease in hemoglobin level, a surgical opinion was sought. When she was evaluated by the vascular surgery team, she had occasional flank pain, but was otherwise stable, and a year had already passed since hemoglobin dropped. As such, a decision was made to maintain the initial approach and manage the patient conservatively.
Conclusion
This case describes an underreported disorder that presents with nonspecific symptoms, demonstrating the difficulties of the diagnostic approach of NCS, as well as the challenges in the appropriate management, given the lack of standardized treatment.
Declaration of patient consent
The author certify that he has obtained appropriate patient consent. Patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published, and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Method of Anatomy.Williams & Wilkins, Baltimore 1937:158-9.
- Nutcracker syndrome: An update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017;53:886-94.
- [Google Scholar]
- An easily missed diagnosis: Flank pain and nutcracker syndrome. BMJ Case Rep 2013:3-5.
- [Google Scholar]







