Translate this page into:
Validity of nutrition risk index as a malnutrition screening tool compared with subjective global assessment in end-stage renal disease patients on peritoneal dialysis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We undertook this study to compare subjective global assessment (SGA) and nutrition risk index (NRI) as malnutrition screening tools in peritoneal dialysis (PD) patients. Nutrition status of the patients was categorized into low, moderate and high risk of malnutrition based on both NRI and SGA. The sensitivity, specificity and predictive values of NRI were compared with SGA, an already validated tool of nutrition status assessment in PD patients. Two hundred and eighty-three end-stage renal disease patients (age 50.02 ± 13.76 years; 204 males, 150 diabetic) were included. Based on SGA, 71/283 (25.08%) had normal nutrition, 192/283 (67.84%) mild–moderate and 20/283 (7.07%) severe malnutrition. Based on NRI, 38/283 (13.43%) patients had normal nutritional status, 193/283 (68.20%) mild-moderate and 52/283 (18.37%) severe malnutrition. Twenty-three of 283 (8.1%) were correctly classified as normal by NRI (true negative) and 197/283 (69.6%) as malnourished (true positive), 15/283 (5.3%) as false negative, 48/283 (16.96%) were misclassified as malnourished (false positive). NRI has sensitivity of 92.9% and specificity of 32.39%. Positive predictive value and Negative predictive values (NPVs) of NRI are 80.41% and 60.53%, respectively. Accuracy of the test is 78%. The receiver operating characteristic curve of NRI is 0.63. To conclude, NRI carries high sensitivity but low specificity as compared to SGA. It can be used as screening tool but not as a diagnostic tool for assessment of nutritional status in PD patients because of its low specificity and NPV.
Keywords
Malnutrition
nutrition risk index
peritoneal dialysis
subjective global assessment
Introduction
Nutrition status assessment is important in the detection of protein energy malnutrition (PEM), dietary requirements, and the development of the alternative nutritional therapies in chronic kidney disease patients.[1] Assessment of nutrition status has assumed greater importance because of the association of malnutrition with increasing morbidity and mortality.[234] Malnutrition is a strong predictor of mortality and morbidity in maintenance peritoneal dialysis (PD) patients.[567] Studies have shown that prevalence of mild to moderate malnutrition varies from 26% to 68% and severe malnutrition from 2% to 8% in PD patients in different parts of the world with high prevalence in developing countries than that of the developed countries.[5678] We have previously shown that the prevalence of malnutrition is high at initiation of PD, and periodic assessment of nutrition status and dietary counseling help in improving the nutrition status of PD patients.[8]
There are many tools for the assessment of nutrition status. Studies have consistently revealed the inadequacy of any single assessment method or tool to assess the nutrition status of patients. Although subjective global assessment (SGA) is a valid estimate of nutrition status of PD patients;[1] individual measurements of parameters often have limited its value in accurately determining the nutrition risk of these patients.[9] Nutrition risk index (NRI), developed by veterans affairs total parenteral nutrition cooperative study group was found to be sensitive, specific and positive predictor for identifying patients with risk of complications after surgery.[10] We have used NRI for estimating nutrition status of PD patients in one of our studies.[11] Recently, Szeto et al.,[12] have used a modified NRI formula for nutrition assessment of geriatric PD patients. However, original NRI formula has never been used and validated for the assessment of nutrition status in large cohort of nongeriatric PD patients. We undertook this study to compare NRI and SGA in large cohort of PD patients, and to determine the sensitivity, specificity and predictive values of NRI compared to SGA and validate its utility as screening tool for nutrition status.
Patients and Methods
During the study period (January 2009 to July 2012), a total of 323 end-stage renal disease (ESRD) patients were started on PD at our institute. Patients <12 years (n = 8) and >65 years (n = 22) in age, those who did not continue PD for 3 months (n = 4), and those who did not consent for the inclusion in the study (n = 6) were excluded. Thus, 283 PD patients remained for analysis. All patients were subjected to detailed history and clinical examination. Nutritional indices were assessed by anthropometry, 72-h dietary diary, body mass index, serum albumin, NRI and SGA. Anthropometric measures and nutrition indices were assessed after 1-month of start of PD.
Assessment of subjective global assessment
All patients were subjected to SGA.[8] We used a 7-point Likert-type scale of four items: weight loss, anorexia, subcutaneous fat and muscle mass. Each item was given scores to produce a global assessment score. Scores of 1–2 represented severe malnutrition; 3–5 mild-moderate malnutrition; and 6–7, normal nutrition. Patients were subdivided into three groups based on SGA score: normal nutrition, mild-moderate malnutrition, and severe malnutrition as previously reported in CANUSA PD Study Group.[7]
Assessment of nutrition risk index
Nutrition risk index was calculated as follows: NRI = (1.519 × serum albumin (g/L) +41.7× (present weight/usual weight). The patients with NRI score of >100 was considered in no risk group, 97.5–100 mild risk, 83.5–97.5 moderate risk, and < 83.5 has severe risk groups.[10] The usual body weight was defined as stable body weight for last 6 months, and the patient's weight was obtained through history or previous measurements, considered to be stable over time.
The sensitivity, specificity, and predictive values of NRI considering SGA as the gold standard were calculated. The patient survival and number of hospitalization of the patients were compared in three risk groups based on NRI and SGA. The receiver operating characteristic (ROC) curves were generated for NRI for our patient population with the use of the SGA as the reference standard. The area under the ROC curve indicated the probability of discriminating a nutritional risk. The cutoff risk point of nutrition for the reference standard was then defined from the highest sensitivity (1-specificity) value in the ROC curve.
The patients were followed until the death of the patient, technique failure, and shift on hemodialysis or, the end of the study period. The study was approved from the ethics committee of Institute.
Statistical analysis
Data are expressed as mean ± standard deviation. Chi-square test was used to compare the proportion between two groups. Student's test was used to compare the means between different groups. A contingency table was used to determine the sensitivity, specificity, predictive values and accuracy of the NRI as a malnutrition screening tool for PD patients compared to SGA. Kaplan Meier survival analysis was used to analyze the survival of patients in three risk groups based on SGA and NRI, and log rank test was used to test the significance. Simple correlations are reported as the Pearson correlations. Statistical significance was reported at P < 0.05. Data were analyzed using SPSS.11 statistical software (SPSS Inc., Chicago, Illinois, USA).
Results
Demographic profile and prevalence of malnutrition
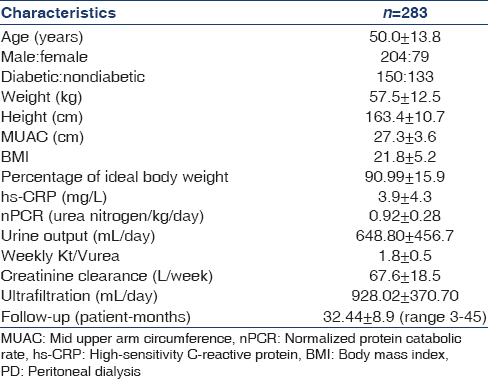
The demographic profile of the patients at the initiation of dialysis is given in Table 1. The mean age of patients was 50.02 ± 13.76 years. The patients were followed up to 32.44 ± 8.9 (range 3–45) patient-months. Of the 283 patients, 150 patients were diabetics and 204 male. On evaluation of peritoneal equilibration tests (PET), 11 (3.9%) had low, 83 (29.3%) of patients low average, 147 (51.9%) high average and 42 (14.8%) patients high (H) transport characteristics. Out of 283 PD patients, 132 (46.6%) patients were vegetarians, 67 (23.7%) were ovo-vegetarians and 84 (29.7%) were nonvegetarians. The mean normalized protein catabolic rate at time of PET was 0.92 ± 0.28 (range 0.41–1.71). The prevalence of malnutrition based on SGA and NRI has been shown in Table 2. Based on SGA, 71/283 (25.08%) had normal nutritional status, 192/283 (67.84%) had mild-moderate malnutrition, and 20/283 (7.07%) had severe malnutrition. On classifying the patients based on NRI, 38/283 (13.43%) patients had normal nutrition status, 193/283 (68.20%) had mild-moderate malnutrition, and 52/283 (18.37%) had severe malnutrition.

The significantly greater numbers of patients with diabetics were detected malnourished based on NRI compared to patients detected malnourished based on SGA. Of the 150 diabetic patients, 122 patients were malnourished and 28 had normal nutrition status based on SGA (P = 0.006) while 136 patients were malnourished and only 14 had normal nutrition status based on NRI (P = 0.024).
Nutrient Intake in different nutrition status groups based on subjective global assessment and nutrition risk index
The mean daily calorie (Kcal/kg/d) intake in patients with normal nutrition status versus mild-moderate malnutrition versus severe malnutrition was 24.7 ± 5.7 versus 17.9 ± 5.4 versus 13.04 ± 4.18, P < 0.001, respectively; and the protein intake (g/Kg/day) was 1.0 ± 0.64 versus 0.8 ± 0.2 versus 0.6 ± 0.6 P < 0.001, respectively based on SGA. Similarly based on NRI, the mean calorie intake (Kcal/kg/d) in patients with normal nutrition status versus mild-moderate malnutrition versus severe malnutrition was 22.8 ± 5.7 versus 19.9 ± 6.5 versus 14.8 ± 4.4, respectively; and the protein intake (g/kg/d) was 0.90 ± 0.3 versus 0.8 ± 0.3 versus 0.6 ± 0.2, respectively. The mean serum albumin level (g/dl) was significantly lower in malnourished patients compared to patients with normal nutrition based on SGA (3.2 ± 0.5 vs. 3.5 ± 0.5 P < 0.001) and NRI (3.1 ± 0.3 vs. 4.0 ± 0.3 P < 0.001) as well.
Risk of hospitalization based on subjective global assessment and nutrition risk index
Of the 212 malnourished patients based on SGA, 138 (65.1%) required hospitalization during follow-up and 74 (34.9%) patients did not require any hospitalization. Of the 138 patients who required hospitalization, 73 (34.4%) patients had multiple hospitalization two or, more times while only 24 (33.8%) patients with normal nutrition based on SGA required hospitalization and 47 (66.2%) did not require any hospitalization. Of the 24 patients who required hospitalization, 13 had two or more than 2 hospitalizations. The relative risk of hospitalization was higher (Relative risk [RR] =3.65; 95% confidence interval [CI] =2.07–6.43; P < 0.001) in malnourished patients compared to patients with normal nutrition status based on SGA.
However, of the 245 malnourished patients based on NRI, 145 (59.2%) required hospitalization and 100 (40.8%) did not required hospitalization while 17 (44.7%) patients with normal nutrition status (n = 37) also required hospitalization and 21 (55.3%) did not required hospitalization. The number of patients who required hospitalization was numerically high in malnourished patients but statistically not significant. The relative risk of hospitalization based on NRI (RR = 1.8, 95% CI = 0.9–3.6, P = 0.068).
Survival of the patients based on nutrition status on subjective global assessment and nutrition risk index
Based on subjective global assessment
The mean survival of the patients with normal nutritional status (33.2 patient-months) was superior to patients with mild-moderate malnutrition (29.3 patient-months) and severe malnutrition (17.8 patient months) based on SGA, P = 0.001. The estimated 1, 2 and 3 years survival rate in these groups was 95%, 77.8%, and 50.1% respectively in patients with normal nutrition status, 89%, 64.9%and 50% in mild–moderate malnourished patients, and 58%, 52.4% and 0% in patients with severe malnutrition. None of the patients with severe malnutrition survived for 3 years based on SGA [Figure 1].

- Kaplan–Meier Survival analysis showing survival of the peritoneal dialysis patients in different groups based on subjective global assessment
Based on nutrition risk index
The mean survival of the patients with normal nutritional status (32 patient-months) was superior to patients with mild-moderate malnutrition (30 patient-months) and severe malnutrition (24.4 patient-months) P = 0.024 based on NRI. The estimated 1, 2 and 3 years survival rate in these groups was 97.1%, 71.6% and 62.7% respectively in patients with normal nutrition status, 89.6%, 71.2%and 50% respectively in mild–moderate malnourished patients and 77.4%, 49.3% and 37% respectively in patients with severe malnutrition [Figure 2].

- Kaplan–Meier Survival analysis showing survival of the peritoneal dialysis patients in different groups based on nutrition risk index
Validity of nutrition risk index
The ability of NRI as a nutrition screening tool to predict nutrition status is shown in Table 3. Totally, 23/283 (8.1%) were correctly classified as being normal/well nourished by NRI screening tool (true negative) and 197/283 (69.6%) of patients were correctly classified as being malnourished (true positive), 15/283 (5.3%) false negative, 48/283 (16.96%) patients were misclassified as being malnourished (false Positive). NRI has a high sensitivity of 92.9% and a low specificity of 32.39%. Positive predictive value of NRI is 80.41%, and negative predictive value (NPV) is 60.53%. Accuracy of the test is 78%. SGA is positively correlated with NRI (r = 0.451, P = 0.01). The ROC curve (sensitivity vs. 1-specificity) of NRI was 0.63 [Figure 3].


- The receiver operating characteristic (ROC) curve of nutrition risk index (NRI) compared to subjective global assessment (The ROC curve [sensitivity vs. 1-specificity] of NRI 1 s 0.63)
Discussion
Bundle of tests varying from simple anthropometry, biochemical parameters, SGA to dual-energy x-ray absorptiometry (DEXA) bio-impedance analysis, and infrared technique based method are used to determine the nutrition status of dialysis patients. SGA has been validated for estimating nutrition status in PD patients and stood the test of time with certain limitations of subjectivity in the test. NRI appears to be simple scoring system for screening malnourished PD patients which can be applied easily and rapidly in a large population with more objectivity compared to SGA.
In this study on the validity of NRI as nutrition status screening tool, we observed that NRI has high sensitivity but low specificity with consideration of SGA as the gold standard for the assessment of nutrition status in PD patients. The NRI has been used to define nutritional risk in a number of recent studies where the effects of under nutrition[13] or nutritional intervention were investigated.[1415] The NRI relies on serum albumin concentration and percentage usual weight. The formulae based calculation of NRI provides some objectivity in the assessment of nutrition status. The formula for NRI also contains serum albumin level which is considered to be one of the important biochemical parameters to assess the nutrition status of PD patients.
Similar to SGA, NRI has been used as a nutrition status tool for the surgical and cancer patients, and it was found to be a sensitive and positive predictor of malnutrition in these patients. However, it has never been used for the ESRD patients on PD. Szeto et al.,[12] have used a modified NRI formulae for the assessment of nutrition status in elderly PD patients. This is the first report on validating the original NRI formula as a malnutrition screening tool compared to SGA in ESRD patients on PD. We observed that NRI underestimated the patients with normal nutrition status (13.43% vs. 25.08%) and over-estimated the severe grades of malnutrition compared to SGA (18.37% vs. 7.07%). The percentage of patients with mild to moderate degree of malnutrition was almost similar by both methods NRI (68.02%) and SGA (67.84%). NRI has the sensitivity of 92.9% and specificity of 32.39%. The ROC curves generated for NRI for our patient population with the use of the SGA as the reference standard, (sensitivity vs. 1-specificity) of NRI was 0.63.
Szeto et al., studied a modified NRI formula, Geriatric Nutrition Risk Index (GNRI) in 314 adult PD[12] patients against their comprehensive malnutrition-inflammation scores (MIS) and 7-point SGA scores. However, they have not found GNRI as a sensitive tool for screening malnutrition and detecting the changes in nutrition status in PD patients. The reason could be the modification from the original formula of NRI. The GNRI was developed by modifying the nutritional risk index for elderly patients. This index is calculated based on serum albumin and body weight, using the following equation: GNRI = (1:4893 × serum albumin [g/dl/d]) + (41:7× [body weight/ideal body weight]). Body-weight to ideal-body-weight ratio was set to 1 when a patient's body weight exceeded the ideal body weight. GNRI was originally meant for the identification of malnutrition in a geriatric population. However, their results remained similar when only patients aged over 65 years were analyzed. However, Yamada et al.[16] found that the GNRI was the simplest and most accurate index for identifying patients on hemodialysis at nutritional risk compared to MIS.
Nutrition risk index predicted hospitalization and survival of PD patients similar to SGA in our study. The NRI has been used to define nutritional risk in a number of recent studies where the effects of undernutrition[13] or nutritional intervention were investigated.[1415] Clugston et al.,[17] found NRI as simple to use and defines a high-risk sub-group of patients with obstructive jaundice. NRI < 83.5 was significantly associated with mortality and longer duration of hospital admission but not complication rate.
As a screening tool, a drawback of the NRI is the reliance on measurements of current and previous body weight, limiting its usefulness where there is a relative increase in body weight due to increase in total body water. The NRI is open to further criticism as a nutrition screening tool for including serum albumin in its formula.[18] Despite these problems and limitations, NRI on admission was shown to predict postoperative complications in surgical patients.[10] Assessing specificity is important in preventing well-nourished patients from being incorrectly identified as malnourished.[19] Finding malnourished patients in need of nutritional intervention will definitely improve the outcome. High sensitivity is the desirable characteristic, and there is no need for very high specificity to screen out malnourished patients.
Conclusion
Nutrition risk index can be used as screening tool for assessment of nutrition status with high sensitivity. However, it cannot be used as a diagnostic tool for assessment of nutritional status in PD patients because of its low specificity and NPV. Further research and multicenter studies are needed using NRI against a broader array of objective and subjective nutritional parameters to confirm its validity as a screening tool for malnutrition in PD patients.
Acknowledgments
We would like to thank Mr. Santosh Kumar Verma for the secretarial assistance and technicians of Renal Laboratory for the support.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Nutritional assessment of continuous ambulatory peritoneal dialysis patients: An international study. Am J Kidney Dis. 1991;17:462-71.
- [Google Scholar]
- The microinflammatory state in uremia: Causes and potential consequences. J Am Soc Nephrol. 2001;12:1549-57.
- [Google Scholar]
- Are there two types of malnutrition in chronic renal failure?. Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953-60.
- [Google Scholar]
- Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996;7:198-207.
- [Google Scholar]
- Changes in nutritional status on follow-up of an incident cohort of continuous ambulatory peritoneal dialysis patients. J Ren Nutr. 2008;18:195-201.
- [Google Scholar]
- Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant. 1993;8:1094-8.
- [Google Scholar]
- Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525-32.
- [Google Scholar]
- Impact of nutritional status on peritonitis in CAPD patients. Perit Dial Int. 2007;27:42-7.
- [Google Scholar]
- Geriatric nutritional risk index as a screening tool for malnutrition in patients on chronic peritoneal dialysis. J Ren Nutr. 2010;20:29-37.
- [Google Scholar]
- Impaired gut barrier function in malnourished patients. Br J Surg. 1996;83:1288-91.
- [Google Scholar]
- A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226:567-77.
- [Google Scholar]
- Two phase randomised controlled clinical trial of postoperative oral dietary supplements in surgical patients. Gut. 1997;40:393-9.
- [Google Scholar]
- Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87:106-13.
- [Google Scholar]
- Nutritional risk index predicts a high-risk population in patients with obstructive jaundice. Clin Nutr. 2006;25:949-54.
- [Google Scholar]
- Symposium on nutrition and surgical practice pre-operative nutritional assessment. Proc Nutr Soc. 1999;58:821-9.
- [Google Scholar]
- Assessment of nutritional status on hospital admission: Nutritional scores. Eur J Clin Nutr. 2003;57:824-31.
- [Google Scholar]







