Translate this page into:
Viral Infection Associated Membranous Nephropathy: Clinical Presentation and Outcomes
*Prabhjot Kaur and Arun Prabhahar are joint first authors
Corresponding author: Raja Ramachandran, Department of Nephrology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. E-mail: drraja1980@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaur P, Prabhahar A, Niranjan AV, Kumar V, Pal D, Rathi M, et al. Viral Infection Associated Membranous Nephropathy: Clinical Presentation and Outcomes. Indian J Nephrol. 2025;35:70-6. doi: 10.25259/IJN_57_2024
Abstract
Background
Viral infections can increase the likelihood of an individual developing membranous nephropathy (MN). Limited information is available regarding the treatment approaches for such cases. We conducted a review focusing on hepatitis B (HBV), hepatitis C (HCV), and human immunodeficiency virus (HIV)-associated MN.
Materials and Methods
Our investigation encompassed patient records and cases documented in the literature, utilizing various search engines (PubMed, Scopus, Embase, and Web of Science). We aimed to identify all reported instances of MN associated with HBV, HCV, or HIV infections between 2010 and February 2023 in individuals aged 18 years and above, who underwent PLA2R testing in their serum or kidney biopsy.
Results
We analyzed 63 patients with MN associated with viral infections, comprising 7 patients from our center and 57 from the review, consisting of 43% with HIV, 28.5% with HBV, 17.5% with HCV, and 11% with mixed infections. The average age of these patients was 47 years. Their mean proteinuria, serum albumin, and creatinine levels were 7.5 g/day, 2.3 g/dl, and 1.4 mg/dl, respectively. Two-thirds of these cases were PLA2R-related. Notably, 24% of patients achieved remission solely through antiviral treatment, while nearly 40% attained remission with a combination of antiviral and immunosuppression therapies. Eight patients did not achieve remission despite receiving immunosuppressive therapy and antiviral agents.
Conclusion
The review suggests that using antiviral medications alone or combined with immunosuppressive therapy can lead to substantial remission in patients with viral-associated MN.
Keywords
Viral infections
Membranous nephropathy
HBV
HCV
HIV
Rituximab
Cyclophosphamide
Corticosteroids
Introduction
Membranous nephropathy (MN) linked to infections has been extensively documented in individuals with human immunodeficiency virus (HIV), as well as hepatitis B (HBV) and C (HCV) infections.1-4 Although the pathophysiology of post-viral MN remains unclear, initial findings indicate that MN might be triggered by viral infection-induced immunomodulation.5 In recent years, a notable transformation in the treatment landscape of viral infections, particularly in the case of Hepatitis C is seen. We anticipate that the successful treatment outcomes may contribute to reducing the incidence of MN associated with these infections.
The molecular categorization of MN has brought about a transformative impact on the disease’s management. Circulating autoantibodies targeting the phospholipase A2 receptor (PLA2R) antigen range between 70% and 80% among individuals with primary MN (PMN).6-9 Initially, the concept of anti-PLA2R antibodies was introduced to differentiate primary MN from secondary forms.10-11 Nevertheless, recent data indicate the presence of anti-PLA2R antibodies in individuals with MN and concurrent viral infections.2,11-15 Despite numerous reports linking viral infections to MN, the question remains unanswered regarding whether remission can be achieved solely through antiviral therapy or if immunosuppressive treatment is also necessary. This manuscript shares our firsthand experiences and presents a case-based review of MN in patients with hepatitis B/C and HIV infection in the post-PLA2R era.
Materials and Methods
Two separate reviewers, PK and AP, conducted literature search across four databases—PubMed, Scopus, Embase, and Web of Science. The aim was to identify all documented cases of MN associated with HBV/HCV/HIV infections between 2010 and February 2023, commonly referred to as the PLA2R era. The inclusion criteria encompassed patients aged 18 years and above who underwent PLA2R testing in either their serum or kidney biopsy. The search utilized specific terms such as ‘Hepatitis virus,’ ‘Hepatitis B,’ ‘HBsAg,’ ‘Hepatitis C,’ ‘HCV,’ ‘Human immunodeficiency virus,’ ‘HIV,’ ‘Viruses,’ ‘Membranous glomerulonephritis,’ ‘MN,’ and ‘Primary membranous nephropathy.’ Further refinement involved additional criteria, including the presence of PLA2R in the title, keywords, or abstract. Including cases that required individual clinical details, investigative findings, and follow-up information. Cohorts and randomized trials that lacked specific patient details were excluded from the analysis. Reviewers independently assessed relevant studies and agreed on including studies that met the specified criteria.
The data are expressed as mean ± standard deviation (SD) and median with interquartile range (IQR) for normally distributed and skewed data, respectively. Categorical data are presented as frequencies or proportions. The data analysis was performed using the Statistical Package for the Social Sciences (SPSS) for Macintosh, version 26.0.0.0, developed by IBM Corp., Armonk, NY, USA. The study was approved by the institutional ethics committee and appropriate patient consent was obtained.
All patients at our center were treated with supportive care, which included angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEi), statins, and anticoagulants (if indicated), along with antiviral therapy (in case of active viral infection).
Patients with inactive infection and persistent proteinuria were offered cyclical cyclophosphamide and corticosteroids in accordance with the KDIGO guidelines (2012).16 For those with contraindications such as diabetes mellitus (DM) or suspected intolerance to the therapy, rituximab therapy was administered, ensuring alignment with the current guidelines set forth by KDIGO (2021).17
Case reports
Case 1
An incidental finding of active HCV infection (viral load: 3890243 IU/ml) was made in a 58-year-old woman during the diagnosis of MN, characterized by proteinuria of 2.8 g/day, serum albumin of 1.6 g/dl, serum creatinine of 0.6 mg/dl, and a PLA2R antibody level of 236 RU/ml. Initially, she underwent treatment with direct-acting antivirals (DAA) for HCV infection, along with supportive therapy involving angiotensin receptor blockers (ARBs), statins, and anticoagulants. However, due to worsening proteinuria and persistent anti-PLA2R positivity despite achieving an undetectable viral load, after 3 months of the disappearance of HCV infection, we initiated her on cyclical cyclophosphamide and corticosteroids (cCYC/CS), as the patient had high-risk disease categorization. After eight months of this regimen, she achieved complete remission [Supplemental Table 1]. Subsequent follow-up, conducted 30 months later, revealed an undetectable viral load, and she remained in a state of complete remission (CR) with negative PLA2R levels.
Case 2
A 45-year-old HIV-positive man and on highly active antiretroviral therapy (HAART) (Abacavir, lamivudine, and dolutegravir) for the past five years (with a CD4 count of 377 cells/uL and an undetectable viral load) was diagnosed with MN during an evaluation for edema. His presentation included 16.9 g/day of proteinuria, serum albumin of 1.7 g/dl, serum creatinine of 1.2 mg/dl, and an anti-PLA2R antibody level of 773.4 RU/ml. Given the absence of HIV replication and a high-risk category, the patient underwent treatment with cyclical cyclophosphamide and corticosteroids (cCYC/CS) within 6 months of diagnosis of membranous nephropathy. He achieved complete remission (CR), as documented in Supplemental Table 1, after four months of cCYC/CS therapy, and viral load was also undetectable. Subsequent follow-up at the end of two years revealed a reduced anti-PLA2R antibody level (2.08 RU/ml), and he maintained a state of complete remission.
Case 3
During the assessment for secondary causes of MN, a sixty-year-old man was detected to have active HCV infection (viral load: 80400 IU/ml). His presentation showed 14.3 g of proteinuria, 1.2 g/dl serum albumin, 2.2 mg/dl serum creatinine, and anti-PLA2R level of 121 RU/ml. Treatment with direct-acting antivirals (DAA) was initiated, resulting in an undetectable viral load three months after diagnosis. Subsequently, the patient was prescribed cyclical cyclophosphamide and corticosteroids (cCYC/CS) for high-risk MN, with anti-PLA2R level at 100 RU/ml.
The patient developed steroid intolerance, marked by elevated blood sugars and urinary tract infection, necessitating a switch to rituximab therapy (two doses of 1 g each). Partial remission (PR) was achieved at ten months, with proteinuria of 3.0 g/day, serum albumin of 3.8 g/dl, and serum creatinine of 1.0 mg/dl. At the 15-month mark, a nephrotic syndrome relapse occurred (proteinuria of 18 g/day, serum albumin of 2.1 g/dl, serum creatinine of 1.3 mg/dl, and PLA2R antibody level of 201 RU/ml). The patient received three additional doses of rituximab (1 g each on days 0, 15, and day 90). Clinical and immunological remission, with anti-PLA2R level of <2.0 RU/ml, was achieved six months after the rituximab injections, and the HCV RNA levels were undetectable. He was in partial remission at 2 years of follow-up.
Case 4
A 70-year-old male was diagnosed with active HCV infection (viral load: 8204946 IU/ml), during the work up of MN (proteinuria 2.9 g/day, serum albumin 2.2 g/dl, serum creatinine 2.1 mg/dl, PLA2R antibody level 12 RU/ml). Despite the undetectable viral load post-DAA, the patient continued to suffer from nephrotic syndrome with kidney dysfunction. He was treated with rituximab therapy (3 doses of 1 g each), considering the patient’s ability to tolerate immunosuppression. He achieved PR in six months. At the last follow-up (22 months from diagnosis), the patient was still in partial remission (proteinuria 1.32 g/day, serum albumin 4.0 g/dl, and serum creatinine 3.0 mg/dl). His anti-PLA2R level was 5.14 RU/ml.
Case 5
A 68-year-old woman with MN and a history of diabetes and hypertension was found to have HBV infection (HBsAg positive, HBeAg negative, and viral load <20 IU/ml). Her initial presentation included proteinuria of 5.2 g/day, serum albumin of 1.5 g/dl, serum creatinine of 0.8 mg/dl, and anti-PLA2R level of 471.8 RU/ml. Additionally, renal vein thrombosis was identified at the time of diagnosis. As the patient had life-threatening complications due to nephrotic syndrome and inactive viral infection, immunosuppressive therapy was started upfront with anti-viral therapy. The treatment involved three doses of Rituximab (1 g each) and Tenofovir Alafenamide to prevent viral reactivation during immunosuppressive therapy. Over six months, the PLA2R antibody titer decreased to 0.6 RU/ml, and she achieved complete remission (CR) at 15 months. Two years later, the patient was diagnosed with vestibular schwannoma, which was successfully removed.
Case 6
A 32-year-old woman was diagnosed with MN, also tested positive for HBV infection (HBsAg positive, HBeAg negative, and an undetectable viral load). Her initial presentation included proteinuria of 2.1 g/day, serum albumin of 2.9 g/dl, serum creatinine of 0.9 mg/dl, and anti-PLA2R level of 48.3 RU/ml. The management strategy involved supportive care. In subsequent follow-ups, her serum PLA2R level tested negative (<2.0 RU/ml). Unfortunately, the patient is lost to follow-up, and no additional data is available for discussion.
Case 7
We diagnosed a 50-year-old man with active hepatitis C while undergoing an assessment for secondary causes of MN, with proteinuria of 2.7 g/day, serum albumin of 2.2 g/dl, and serum creatinine of 0.6 mg/dl. Both his serum and tissue PLA2R levels were negative. The treatment approach involved direct-acting antivirals (DAA) and supportive care for MN. He achieved complete remission with an undetectable HCV viral load within six months of diagnosis. At the most recent two-year follow-up, the patient remains in complete remission.
Results
The exploration of PubMed, EMBASE, Medline, and Web of Science databases produced 50,531 records, as illustrated in Figure 1. Including seven patients from our centre contributed to the analysis of 63 cases in this review.1-4,13-14,18-25
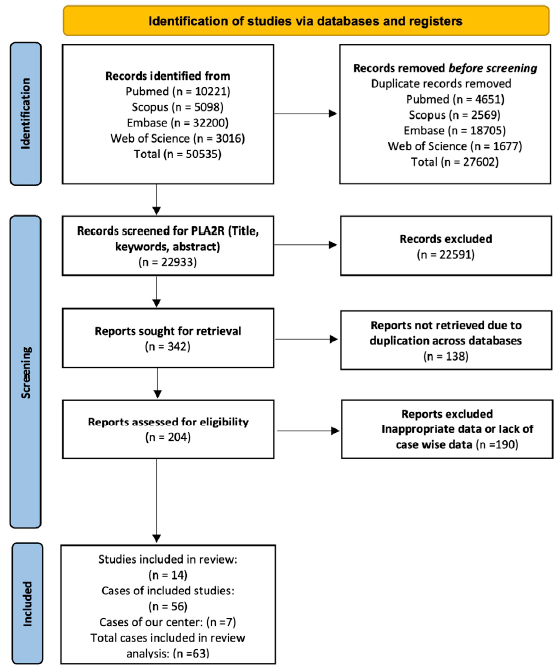
- Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. The diagram illustrates the systematic process of incorporating papers identified through our search. PLA2R: Phospholipase A2 receptor.
The mean age was 47.0 years (SD of 15.40). The male-to-female ratio was 2:1.
The mean proteinuria, serum albumin, and creatinine were 7.5 g/day (38 cases), 2.3 g/dl (40 cases), and 1.4 mg/dl (44 cases), respectively. A total of 27 cases were associated with HIV (43%), 18 with HBV (28.5%), 11 with HCV (17.5%), 4 with both HCV and HIV (6%), and 3 with both HBV and HIV (5%). Among the 63 cases, 39% had underlying comorbidities, including hypertension, diabetes, dyslipidemia, and hypothyroidism, among others. Two cases involved liver disease (cases 32 and 45), and two were associated with malignancies (cases 42 and 60). Notably, in the case series by Ma et al., four patients (cases 16-19) were diagnosed with both syphilis and HIV infection at the time of MN diagnosis.4
At the time of MN diagnosis, one patient (case 25) was pregnant. Antinuclear antibody (ANA) positivity was observed in three patients: two with HIV (cases 44 and 45) and one with HCV (case 34). Case #63, a kidney transplant recipient diagnosed with MN 16 years post-transplant, had an HCV infection. Clinical remission was achieved by 15 patients (24%) through antiviral treatment alone, while 25 patients (40%) required immunosuppressive therapy either independently or in conjunction with antiviral treatment. Upon stratification based on PLA2R positivity, no significant difference was observed for remission rate with antiviral treatment alone and immunosuppressive with and without antivirals between both PLA2R-related and unrelated patients (p = 0.089). Also, no statistically significant difference was observed for the patients who received only antiviral therapy with positive and negative PLA2R antibodies (p = 0.55). The comprehensive details of individual studies’ clinical, biopsy, and treatment aspects can be found in Table 1, and additional in-depth information is available in Supplemental Tables 1 and 2.
| Parameter(s) |
Total (n = 63) |
HBV (n = 18) |
HCV (n = 11) |
HIV (n = 27) |
Mixed infection (n = 07) |
|---|---|---|---|---|---|
| Age | 47.0 ± 15.4 | 43.5 ± 15.1 | 54.1 ± 12.3 | 46.7 ± 15.3 | 46.1 ± 20.2 |
| Male:Female | 42:21 | 7:11 | 8:3 | 21:6 | 6:1 |
| Proteinuria (g/day) | 7.5 ± 4.1 | 7.4 ± 5.0 | 4.9 ± 4.5 | 8.5 ± 3.5 | 7.4 ± 3.2 |
| Serum creatinine (mg/dl) | 1.4 ± 2.4 | 0.7 ± 0.2 | 1.3 ± 0.7 | 2.0 ± 3.4 | 1.2 ± 0.5 |
| Serum albumin (g/dl) | 2.3 ± 0.7 | 2.1 ± 0.5 | 2.0 ± 0.7 | 2.5 ± 0.6 | 3.0 ± 1.4 |
| Tissue PLA2R positivity | 36/52 (68%) | 15/17 (88%) | 9/11 (82%) | 9/19 (47%) | 3/6 (50%) |
| Serum anti-PLA2R positivity | 27/41 (66%) | 13/15 (86%) | 5/9 (55%) | 7/11 (63.6%) | 2/6 (33%) |
| Immunofluorescence | |||||
| IFTA (≥50%) | 4/38 (10.5%) | 0/10 (0%) | 0/7 (0%) | 4/16 (25%) | 0/5 (0%) |
| IgG (≥2+) | 38/59 (64%) | 11/17 (65) | 7/10 (70%) | 17/25 (68%) | 3/6 (50%) |
| IgG4 (≥2+) | 14/32 (44%) | 6/7 (86%) | 2/7 (28%) | 6/15 (40%) | 0/0 (0%) |
| IgM (≥2+) | 7/47 (15%) | 1/16 (6%) | 0/6 (0%) | 5/18 (28%) | 1/6 (16%) |
| IgA (≥2+) | 1/42 (2%) | 0/14 (0%) | 0/5 (0%) | 0/17 (0%) | 1/6 (16%) |
| Electron microscopy | |||||
| Subepithelial deposits | 52/52 (100%) | 15/15 (100%) | 6/6 (100%) | 24/24 (100%) | 7/7 (100%) |
| Subendothelial deposits | 7/33 (21%) | 3/7 (42.8%) | 0/4 (0%) | 3/18 (16.6%) | 1/4 (25%) |
| Intramembranous deposits | 22/36 (61%) | 3/6 (50%) | 3/4 (75%) | 15/22 (68%) | 1/4 (25%) |
| Mesangial deposits | 25/42 (59%) | 9/13 (69%) | 3/5 (60%) | 10/19 (52%) | 3/5 (60%) |
| TRIs deposits | 7/24 (29%) | 3/8 (37%) | 2/4 (50%) | 1/8 (12.5%) | 1/4 (25%) |
| Treatment | |||||
| Anti-viral | 25/63 (40%) | 7/18 (39%) | 1/11 (9%) | 14/27 (52%) | 3/7 (43%) |
| IST | 3/63 (5%) | 0/18 (0%) | 3/11 (27%) | 0/27 (0%) | 0/7 (0%) |
| Anti-viral + IST | 31/63 (49%) | 8/18 (44%) | 7/11 (64%) | 12/27 (44%) | 4/7 (57%) |
| Supportive | 4/63 (6%) | 3/18 (17%) | 0/11 (0%) | 1/27 (4%) | 0/7 (0%) |
| Composite remission | 43/52 (82%) | 17/18 (95%) | 10/11 (91%) | 12/17 (71%) | 4/6 (67%) |
MN: Membranous nephropathy; HBV: Hepatitis B; HCV: Hepatitis C; HIV: Human Immunodeficiency Virus; IgA: Immunoglobulin A, IgG: Immunoglobulin G, IgM: Immunoglobulin M; IST: Immunosuppressive therapy; PLA2R: Phospholipase A2 receptor; IFTA: Interstitial fibrosis and tubular atrophy; TRIs: Tubuloreticular inclusions.
HBV infection-associated MN
Half of the 18 patients with HBV-associated membranous nephropathy (HBV-MN) had an active viral infection at the time of MN diagnosis. In 16 patients (88.8%), MN was found to be PLA2R-associated, as indicated by 13 cases with positive serum results, 15 with positive biopsy findings, and 12 exhibiting positivity in both. The detailed clinical, biopsy, and treatment information, along with outcomes, is provided in Table 1.
Out of the 18 HBV-MN patients, 15 (83%) received treatment with antiviral agents alone (7 cases) or in combination with immunosuppressive (IS) therapy (8 cases), while the remaining three (17%) received supportive care. The antiviral agents administered included entecavir in 11 cases, tenofovir in 3 cases, and lamivudine in 1. Immunosuppressive therapy involved rituximab, tacrolimus, and cyclophosphamide in 4, 5, and 2 patients, respectively. Remarkably, 95% of the patients (17 individuals) achieved remission, with 53% in complete remission (CR) and 47% in partial remission (PR). Only one patient did not achieve remission.
During the follow-up period, two patients treated with immunosuppressive therapy (cases #57 and #26) experienced complications, including renal vein thrombosis, pulmonary embolism, and crescentic transformation. In another case (#9), a patient treated with tacrolimus received rituximab and cyclophosphamide. Detailed clinical information and outcome data are in Supplemental Tables 1 and 2.
HCV infection-associated MN
There were 11 individuals diagnosed with HCV-associated membranous nephropathy, with 63.6% (7 patients) having a detectable viral load at the time of diagnosis. In contrast, 36.4% (4 patients) had an undetectable viral load with historical evidence of infection, as indicated in the Nikolopoulou et al. study (#31-34)).2 Nine patients, constituting 82% of the cohort, tested positive for PLA2R, with 5 in serum, 9 in biopsy, and 5 in both. Among individuals with HCV-associated membranous nephropathy (HCV-MN), eight (73%) underwent treatment with either antiviral therapy alone (1 case) or in combination with immunosuppression (7 cases). At the same time, three patients received only immunosuppressive therapy (IST). The primary immunosuppressive drugs included Rituximab (5 cases), tacrolimus (4 cases), and cyclophosphamide (3 cases), either alone or in various combinations.
Remarkably, 91% of patients (all except #33) achieved clinical remission, with 30% in complete remission (CR), 50% in partial remission (PR), and the type of remission not specified in 20% (2 cases). Following the second transplant, one patient (#60) experienced a recurrence of MN associated with HCV infection, leading to the loss of both the native and first transplant kidneys due to fulminant MN. Detailed clinical data can be found in Table 1, while patient-wise information is presented in Supplemental Tables 1 and 2.
HIV infection-associated MN
Within the group of 27 patients with HIV-associated membranous nephropathy, 30.7% exhibited a detectable viral load at the time of diagnosis, while data for one patient (#49) was unavailable. PLA2R-associated MN was identified in 14 patients (54%), distributed across 7 cases in serum, 9 in biopsy, and 2 in both. However, information regarding PLA2R status was not accessible for one case (#46). Of the cohort, 96% (26 patients) received Highly Active Antiretroviral Therapy (HAART), either alone (14 cases) or in conjunction with immunosuppressive (IS) therapy (12 cases). One patient (#35) (4%) received supportive care exclusively. The most commonly utilized immunosuppressive medications included Rituximab (5 cases), tacrolimus (4 cases), cyclophosphamide (5 cases), and prednisone (1 case). Overall, 71% of patients achieved remission (comprising 7 in complete remission and 5 in partial remission), while 29% did not achieve remission, and outcome data for 10 patients were unavailable. In-depth details are presented in Supplemental Tables 1 and 2.
Co-infections-associated MN
There were seven patients with dual viral infections, comprising four cases with both HIV and HCV and three cases with HIV and HBV co-infections. In 28.5% of these cases, the viral load was detectable. MN was found to be PLA2R-associated in 43% of patients (involving 2 cases in serum, 3 in biopsy, and 2 in both). All seven patients underwent treatment with HAART therapy, either alone (3 cases) or in combination with immunosuppressive therapy (IST) (4 cases). The immunosuppressive regimen included rituximab and ACTH gel in one case each and tacrolimus in two cases. Notably, 67% of patients (based on available data from 6 cases) achieved clinical remission. Patient-specific clinical data is provided in Supplemental Tables 1 and 2.
Discussion
We highlight the close link netween MN and prevalent viral infections. Over a third of the patients exhibited active viral replication during the diagnosis, and the PLA2R association was identified in two-thirds of the cases. The administration of antiviral therapy, with or without concurrent immunosuppressive treatment, led to remission in over three-fourths of the cases. Overall, individuals with MN associated with viral diseases demonstrated a favorable prognosis.
MN is traditionally categorized into primary and secondary MN. Cases lacking associations with drugs, infections, or other systemic diseases are termed primary MN. Identifying the PLA2R antigen has enhanced our comprehension of MN and has been utilized as a tool to distinguish between primary and secondary MN. However, recent reports have indicated that PLA2R is linked to autoimmune diseases such as lupus nephritis, sarcoidosis, infections, and malignancies.1-4,15,26-29 According to the present analysis, approximately two-thirds of viral-associated MN cases were found to be PLA2R-related, displaying a heightened association with hepatitis B/C infection (exceeding 80%). This challenges our conventional understanding of primary and secondary MN, raising uncertainties about whether viral infection acts as a trigger or merely assumes a coincidental role.
Viruses can potentially contribute to the predisposition of MN through various mechanisms. The investigation conducted by Xie and colleagues uncovered the existence of anti-PLA2R antibodies and observed an overlap between PLA2R and HbsAg along capillary walls.12 A recent study found that introducing the Hepatitis B virus into human podocytes induced PLA2R antigen expression.30 Indirect pathways involve the disturbance of host defense mechanisms by pathogen-associated molecular patterns (PAMPs) and the entrapment of circulating immunoglobulin complexes, resulting in inflammation. A sustained inflammatory response can further activate non-specific auto-reactive lymphocyte clones. Additionally, the release of interferon I by NLR family pyrin domain-containing protein 3 (NLRP3) may contribute to podocyte loss.5 Hence, it is plausible that both direct and indirect mechanisms generate anti-PLA2R (or other autoantibodies) antibodies during viral infections. The persistence of clinical symptoms despite viral replication suppression implies the involvement of multiple mechanisms in the pathogenesis of virus-associated MN. The double-hit hypothesis may contribute to disease pathogenesis, involving genetic susceptibility as the first hit, followed by the direct or indirect impact of the virus on antibody production.31 In the current review, only a quarter of patients with active MN attained remission with antiviral therapy, supporting the double-hit hypothesis.
As indicated in Supplemental Table 3, the initial parameters of the current study, including proteinuria, serum creatinine and albumin levels, and anti-PLA2R status, closely resemble those observed in prior studies such as MENTOR, STARMEN, and RI-CYCLO.32-34 More than half of the individuals with viral disease-associated MN exhibited mesangial proliferation and intramembranous deposits, features not typical of primary MN. This may be partly attributed to the viral infection inducing proliferative changes. Compared to the other two groups, two-thirds of patients with MN associated with HCV infection required immunosuppressive treatment. This is partly because direct-acting antiviral agents (DAAs) generally yield a sustained virological response without the risk of viral reactivation upon treatment discontinuation, making the addition of immunosuppressive agents convenient. Patients with HBV/HCV-associated MN demonstrated higher remission rates than those with HIV or combined infections.
The treatment approach for viral-associated MN lacks clarity, specifically regarding the use of immunosuppressive therapy, the timing of its administration, and the appropriate dosage. In the current study, no significant difference in remission rates was observed between patients undergoing antiviral therapy and those receiving immunosuppressive therapy. However, it’s important to note that a direct comparison between these groups is challenging, as patients who did not respond to antiviral therapy might have subsequently received additional immunosuppressive treatments. It has been previously suggested that patients with viral disease-associated MN may benefit from an extended course of antiviral therapy.1 The findings from this analysis indicate that immunosuppressive therapy is safe and effective for individuals with viral-associated MN. This is especially when employed in conjunction with antiviral treatments for cases involving HBV and HIV or post-sustained virological response (with DAAs) in patients infected with HCV.
To the best of our knowledge, this is the first series that thoroughly examines data from all documented instances of membranous nephropathy associated with hepatitis B/C and HIV. However, it is essential to acknowledge certain limitations in this study, including a relatively small sample size, the absence of a standardized approach in managing cases, and some missing data in a few instances.
The findings of the present study indicate that two-thirds of MN linked to common viral diseases are PLA2R-associated and combination of antiviral therapy, with or without immunosuppressive therapy, achieves remission in three-quarters of the cases.
Acknowledgment
The work was supported by a grant from the Indian Council of Medical Research, New Delhi, India, to Dr. Raja Ramachandran.
Financial support and sponsorship
The present work was supported by the Indian Council of Medical Research (ICMR), No 5/4/7-2/Nephrology/2021/NIC-II.
Conflicts of interest
There are no conflicts of interest.
References
- Favorable outcome in PLA2R positive HBV-associated membranous nephropathy. BMC Nephrol. 2022;23:246.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Membranous nephropathy associated with viral infection. Clin Kidney J. 2021;14:876-83.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Membranous nephropathy in patients with HIV: a report of 11 cases. BMC Nephrol. 2020;21:401.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The spectrum of kidney biopsy findings in Chinese HIV-infected patients. HIV Med. 2022;23(Suppl 1):23-31.
- [CrossRef] [PubMed] [Google Scholar]
- Glomerulonephritis: immunopathogenesis and immunotherapy. Nat Rev Immunol. 2023;23:453-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol Dial Transplant. 2016;31:1486-93.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of PLA2R antibody in treatment of membranous nephropathy. Kidney Int Rep. 2018;3:498-501.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96:1292-302.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797-804.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709-15.
- [CrossRef] [PubMed] [Google Scholar]
- Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol. 2015;41:345-53.
- [CrossRef] [PubMed] [Google Scholar]
- PLA2R related primary membranous nephropathy in a hepatitis C positive patient. Nephrology (Carlton). 2018;23:288.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of rituximab in hepatitis b virus-associated PLA2R-positive membranous nephropathy. Kidney Int Rep. 2018;3:486-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Secondary membranous nephropathy. A narrative review. Front Med (Lausanne). 2020;7:611317.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139-274.
- [Google Scholar]
- KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1-S276.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of HIV-associated lupus-like membranous nephropathy with tacrolimus: A case report and review of the literature. Life (Basel). 2023;13:641.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- NELL1-Positive HIV-associated lupus-like membranous nephropathy with spontaneous remission. Glomerular Dis. 2022;2:184-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Primary membranous glomerulonephritis with negative serum PLA2R in haemophilia A successfully managed with rituximab - case report and review of the literature. BMC Nephrol. 2021;22:268.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rituximab for lupus-like membranous nephropathy in the setting of well-controlled HIV infection. Am J Ther. 2020;28:e732-e4.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-phospholipase A2 receptor antibody positive hepatitis B virus-associated membranous nephropathy remitted with entecavir after relapse with lamivudine. J Nephropathol. 2018;7:5.
- [Google Scholar]
- Recurrent membranous nephropathy and acute cellular rejection in a patient treated with direct anti-HCV therapy (ledipasvir/sofosbuvir) Transpl Infect Dis. 2018;20:e12959.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Idiopathic membranous nephropathy: Diagnostic and therapeutic challenges. Am J Nephrol. 2016;43:65-70.
- [CrossRef] [PubMed] [Google Scholar]
- Phospholipase A2 receptor positive membranous nephropathy long after living donor kidney transplantation between identical twins. Nephrology (Carlton). 2015;20(Suppl 2):101-4.
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies to M-type phospholipase A2 receptor (PLA(2)R) in membranous lupus nephritis. Lupus. 2019;28:396-405.
- [CrossRef] [PubMed] [Google Scholar]
- Phospholipase A2 receptor and sarcoidosis-associated membranous nephropathy. Nephrol Dial Transplant. 2015;30:1047-50.
- [CrossRef] [PubMed] [Google Scholar]
- Malignancy-associated membranous nephropathy with positive anti-PLA2R autoantibodies: Coincidence or connection. Case Rep Nephrol Dial. 2021;11:334-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021;99:967-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HBx-induced PLA(2)R overexpression mediates podocyte pyroptosis through the ROS-NLRP3 signaling pathway. Ren Fail. 2023;45:2170808.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The etiology of glomerulonephritis: Roles of infection and autoimmunity. Kidney Int. 2014;86:905-14.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36-46.
- [CrossRef] [PubMed] [Google Scholar]
- The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99:986-98.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab or cyclophosphamide in the treatment of membranous nephropathy: The RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32:972-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







