Translate this page into:
What could be the expected solute clearance with single exchange of icodextrin?
Address for correspondence: Dr. Tarun K. Jeloka, Department of Nephrology, Aditya Birla Memorial Hospital, Thergaon, Pune - 411 033, Maharashtra, India. E-mail: tjeloka@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Icodextrin-based solutions are better in terms of ultrafiltration and solute removal compared to glucose-based dialysis solution in peritoneal dialysis (PD) patients.[12345] However, individual contribution of icodextrin on solute clearance is difficult to ascertain because of the influence of concomitant glucose-based dialysate exchanges and the effect of dilution with residual volume of previous exchange.[6] We analyzed our data of “Ico-alone” incremental dialysis patients to ascertain the solute clearance of single icodextrin exchange in PD patients,[78] uninfluenced by concomitant glucose exchanges.
This was a post-hoc analysis of patients on “Ico-alone” incremental dialysis.[8] All adult patients with significant residual renal function, opting for PD, underwent measurement of urinary KT/V before commencement of PD. Those having a urinary KT/V of about one were offered incremental dialysis and initiation with single nocturnal icodextrin exchange – “Ico-alone” group.[8] All others were initiated with conventional PD (3 exchanges of 2 L standard glucose-based dialysate). Adequacy was done at 1, 3 and 6 months and then 6 monthly. Adequacy test was done using PD Adequest 2 software (Baxter Healthcare Corporation, USA) computer kinetic model for individual patients. Predialysis urinary KT/V was calculated by the same software with blank (zero) peritoneal dialysate reports. Target adequacy was kept as weekly KT/V urea >1.7. Patients in “Ico-alone” group, falling short of adequacy or if clinically indicated with oliguria and/or fluid overload, were shifted to conventional PD. Patients were followed every month for clinical and biochemical examinations. Mean dialysate (icodextrin) solute clearance, KT/V urea, was determined from all the adequacy tests available over the period.
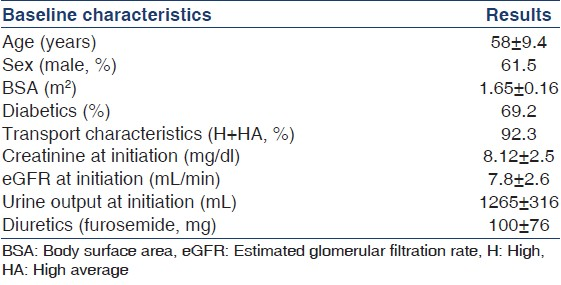
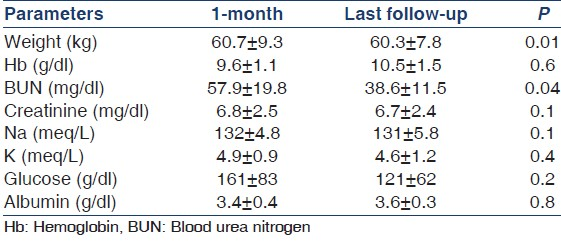
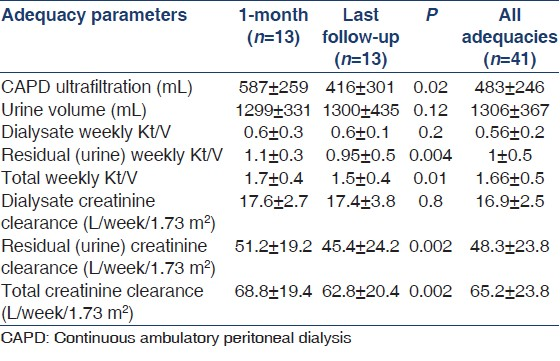
A total of 13 patients satisfied the criteria and were initiated on “Ico-alone” incremental dialysis protocol. Baseline characteristics of study patients are shown in Table 1 and laboratory parameters in Table 2. Table 3 shows the adequacy at 1 month, at last follow-up and means of all adequacies available over the 5-year period. Mean age was 58 ± 9.4 years with 38.4% males and 69.2% diabetics. Mean dwell time was 11.9 ± 0.4 h. Mean ultrafiltration was 483 ± 246 ml (41 adequacies). Median period on “Ico-alone” protocol was 9.6 months. Mean total KT/V at 1 month, at last follow-up and of all the adequacies available over the 5 years were 1.7 ± 0.4, 1.5 ± 0.4 and 1.66 ± 0.5, respectively. Mean dialysate (icodextrin) KT/V at 1 month, at last follow-up and of all adequacies available were 0.6 ± 0.3 (n = 13), 0.6 + 0.1 (n = 13) and 0.56 ± 0.2 (n = 41), respectively. There was no change in icodextrin solute clearance over the study period as shown by the adequacy tests. Those converted to conventional dialysis were due to drop in residual renal clearance rather than change in icodextrin clearance over the period.
Several studies are available looking at the ultrafiltration with icodextrin. However, solute clearance with icodextrin has not been specifically looked at. The available literature shows solute clearance with icodextrin to be about 0.31 KT/V.[3] However, practically, icodextrin clearance is confounded by use of concomitant glucose solutions. The contribution of icodextrin to the total solute clearance is affected by clearances of previous glucose dialysate exchanges as well as the dilution of icodextrin from possible residual dialysate of previous exchange (peritoneal residual volume). Peritoneal residual volume has been studied and noted as an important cause of decreased ultrafiltration,[6] but it can for the same reason, decrease solute clearance as well.[910] As our study was on single icodextrin exchange, they truly represent the solute clearance of icodextrin without influence of glucose dialysate as in conventional PD regime.
We found that single exchange of icodextrin can give a clearance of about 0.6 KT/V urea as against 0.3 mentioned in the literature. We also demonstrated that icodextrin clearance does not decrease over time and remains static at about 0.6 KT/V. As noticed, the ultrafiltration volume was 587 ± 259 at the beginning of the study and later was 416 ± 301 at the end of the study period (P = 0.02). However, KT/V remained similar at these points, that is, 0.6 ± 0.3 and 0.6 ± 0.1 (P = 0.2). The difference in icodextrin clearance as compared to other studies mentioned could be due to difference in “V” or as hypothesized “dilution” with the residual volume in conventional regime, thereby decreasing the real potential of icodextrin. The average body weight and body surface area of our patients in this study was 60.7 ± 9.3 kg and 1.65 ± 0.16 m2, respectively, which may be smaller than western population. Hence, we need more studies from our part of the world to ascertain the clearance of icodextrin in our patients and whether the difference is due to the volume of distribution “V” or because of the dilution in conventional regime. To conclude, clearance from single exchange of icodextrin in our patient population is about 0.6.
References
- A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter investigation of icodextrin in ambulatory peritoneal dialysis. Kidney Int. 1994;46:496-503.
- [Google Scholar]
- Icodextrin Study Group. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. 2002;40:1055-65.
- [Google Scholar]
- Comparison of icodextrin and glucose solutions for the daytime dwell in automated peritoneal dialysis. Nephrol Dial Transplant. 1999;14:1530-5.
- [Google Scholar]
- Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. 2005;16:546-54.
- [Google Scholar]
- Randomized controlled trial of icodextrin versus glucose containing peritoneal dialysis fluid. Clin J Am Soc Nephrol. 2009;4:1799-804.
- [Google Scholar]
- Peritoneal residual volume induces variability of ultrafiltration with icodextrin. Perit Dial Int. 2014;34:95-9.
- [Google Scholar]
- "Icodextrin alone" for initiation of peritoneal dialysis. Perit Dial Int. 2008;28:563-4.
- [Google Scholar]
- "Ico-Alone" single nocturnal exchange to initiate peritoneal dialysis in patients with residual renal function-Five year, single centre experience. Indian J Nephrol. 2013;23:276-9.
- [Google Scholar]
- High peritoneal residual volume decreases the efficiency of peritoneal dialysis. Kidney Int. 1999;55:2040-8.
- [Google Scholar]
- Residual volume in continuous ambulatory peritoneal dialysis patients from various solute calculations. Adv Perit Dial. 1999;15:167-70.
- [Google Scholar]






