Translate this page into:
Wilson’s Disease Presents as Recurrent Hypokalemic Muscle Paralysis
Address for correspondence: Dr. Mythri Shankar, Assistant Professor, Department of Nephrology, Institute of Nephro-Urology, Bengaluru, Karnataka, India. E-mail: mythri.nish@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 21-year-old euthyroid gentleman born to nonconsanguineous parents was diagnosed with bipolar affective disorder. He presented 4 years later with hypokalemic quadriparesis. On evaluation, he was found to have features of both proximal and distal renal tubular acidosis. Ophthalmologic examination by slit lamp confirmed the presence of the Kayser–Fleischer ring. The diagnosis of Wilson’s disease was established with serum ceruloplasmin levels and 24-h urinary copper levels.Here is a rare clinical presentation of Wilson’s disease in the form of hypokalemic muscle paralysis due to proximal renal tubular acidosis with distal tubule involvement. The diagnosis was delayed due to the initial presentation with psychiatric symptoms.
Keywords
Eye
kidney
Wilson’s
Background
Wilson’s disease[1] is caused due to a rare genetic autosomal recessive mutation of ATP7B on chromosome 13q14-21. It has a prevalence of one in 30,000 live births. ATP7B gene codes for an intracellular copper transporter. It usually presents with neuropsychiatric or hepatic symptoms. Rare clinical presentations include hemolytic anemia, bone pathologies, and renal calculi.[2] Also, it rarely presents with renal tubular acidosis, either proximal or distal.[34] Here, we present, to the best of our knowledge, the first case report of Wilson’s disease in the world, presenting as hypokalemic quadriparesis due to both proximal and distal renal tubular acidosis.
Case Report
A 21-year-old gentleman born to nonconsanguineous parents from West Bengal was diagnosed with bipolar affective disorder and was started on olanzapine and fluoxetine. He presented to us 4 years later with complaints of severe muscle weakness and fatigue. On further questioning, he gave a history of muscle weakness treated with potassium supplements 1 year back. However, he had stopped all medications for 9 months. There was no pain or sensory abnormalities. He denied smoking, alcohol consumption, or any other forms of drug abuse. There was no significant family history.
On examination, he was conscious and oriented, but agitated. Vitals were within normal limits with blood pressure of 122/66 mmHg and a normal heart rate of 72 beats/min. Upper and lower limbs had a power of 2/5, with diminished patellar and ankle reflexes. Plantars were mute bilaterally. There was no evidence of bulbar, respiratory, or sphincter muscle involvement. There was no defined sensory level. There was no organomegaly. Examination of other systems was unremarkable.
On laboratory evaluation, complete blood count was as follows: hemoglobin- 15.4 g%, total white blood cell (WBC) count- 5550 cells/mm3, and platelet count- 1.27 lakhs/mm3. Renal function tests and electrolytes were as follows: serum (s.) creatinine- 1.08 mg/dl, blood (b.) urea- 29 mg/dl, s. sodium- 122 mEq/l, s. potassium- 1.5 mEq/l, s. chloride- 96 mEq/l, s. bicarbonate- 17 mEq/l, s. calcium- 8.5 mg/dl, s. phosphorous- 1.55 mg/dl, s. magnesium- 1.5 mg/dl, random blood sugar- 108 mg/dl, and s. uric acid- 3.1 mg/dl. Other labs showed thyroid stimulating hormone (TSH)- 4.7 mIU/l, intermediate parathyroid hormone (iPTH)- 29 pg/ml (normal range 15–68.3 pg/ml), and vitamin D- 27.8 ng/ml. Glycated hemoglobin (HbA1c) was 4.8%. Liver functions tests were normal. Electrocardiogram (ECG) [Figure 1] showed QT prolongation, flattening of T waves, and ST depression, which are features suggestive of hypokalemia. Also, his 24-h urine output was 4100 ml. Urine dipstick showed pH- 6.6, glucose- nil, and protein- 1+. The 24-h urine protein was 640 mg. Urine routine and microscopy revealed bland sediments. Arterial blood gases were as follows: pH- 7.3, bicarbonate (HCO3)- 15.3 mEq/l, partial pressure of carbon dioxide (pCO2)- 30.1 mmHg.
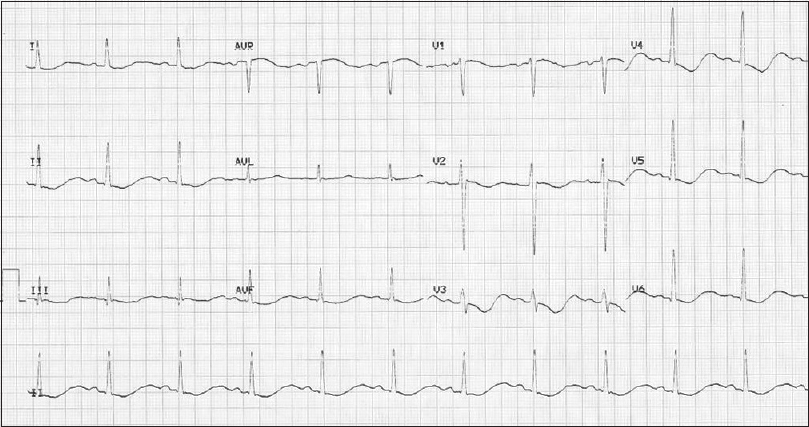
- ECG shows QT prolongation, flattening of T waves, and ST depression. ECG = electrocardiogram
The 24-h urine electrolytes were as follows: potassium- 62 mEq/24 h (25–125), sodium- 198 mmol/24 h (40–220), magnesium- 81 mg/24 h (73–122), phosphorous- 355 mg/24 h (400–1300), calcium- 211 mg/24 h (100–300), citrate 470 mg/24 h (>640 mg/24 h), creatinine- 1291 mg/24 h, and uric acid- 690 mg/24 h (250–750). Spot urine electrolyte values were as follows: urine sodium- 50.9 mEq/l, urine potassium- 15.9 mEq/l, and urine chloride- 56.4 mEq/l. Compensated non-anion gap metabolic acidosis was confirmed. Urinary anion gap: (urine sodium + urine potassium) − urine chloride = (50.9 + 15.9) − 56.4 was 10.4. The presence of urine anion gap is suggestive of renal tubular acidosis. Urine pH of 6.6 (>6) is suggestive of Type 1 or distal renal tubular acidosis. Hypocitraturia (24-h urinary citrate excretion <500 mg), hypercalciuria (calcium/creatinine ratio 0.02, ratio of >0.01 is suggestive of hypercalciuria), and dilute urine (defective concentration ability) are all features of distal renal tubular acidosis.
Proteinuria and increased fractional excretion of phosphate (19.2%), calcium (calcium/creatinine ratio = 0.02), and uric acid (18.62%) are suggestive of proximal renal tubular dysfunction. Increased fractional excretion of magnesium (6.3%) is suggestive of renal loss, predominantly defect in the loop of Henle. Also, this patient had hypokalemia with increased fractional excretion of potassium (FEK; 35.12%), which is a feature of both Type 1 and Type 2 renal tubular acidosis. Studies have shown that the FEK value in hypokalemic patients of extrarenal origin generally ranges from 1.5% to 6.4%, whereas FEK in patients with hypokalemia of renal origin is above 9.5%. Hence, here, it is suggestive of both proximal and distal renal loss.
He was admitted to the intensive care unit for intravenous correction of potassium via a central line. He also received intravenous magnesium sulfate correction. After intravenous correction, he was switched over to oral potassium magnesium citrate supplements.
Antinuclear antibody (ANA) profile and serum for protein electrophoresis were negative.
Ultrasound showed normal echotexture of the liver. There was no evidence of renal calculi. Kidney size was as follows: right kidney 9.8 cm and left kidney 10.1 cm. Eye examination revealed Kayser–Fleischer (KF) rings, which was confirmed on slit lamp [Figure 2]. The diagnosis of Wilson’s disease was established with s. ceruloplasmin level of 0.18 g/l (normal 0.2–0.6 g/l), presence of KF rings, and high 24-h urine copper excretion of 104 mcg (normal 10–30 mcg/24 h) [Figure 3]. No genetic studies were performed due to financial constraints.
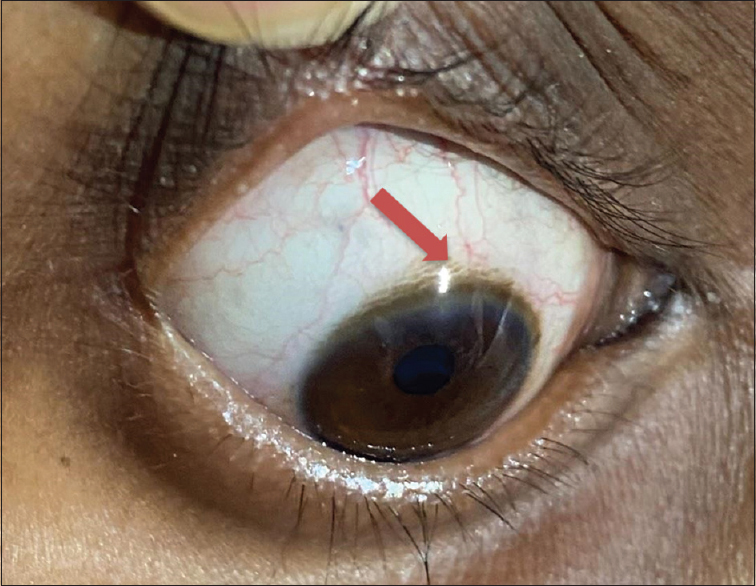
- Arrow shows Kayser–Fleischer rings seen at the limbus of the cornea
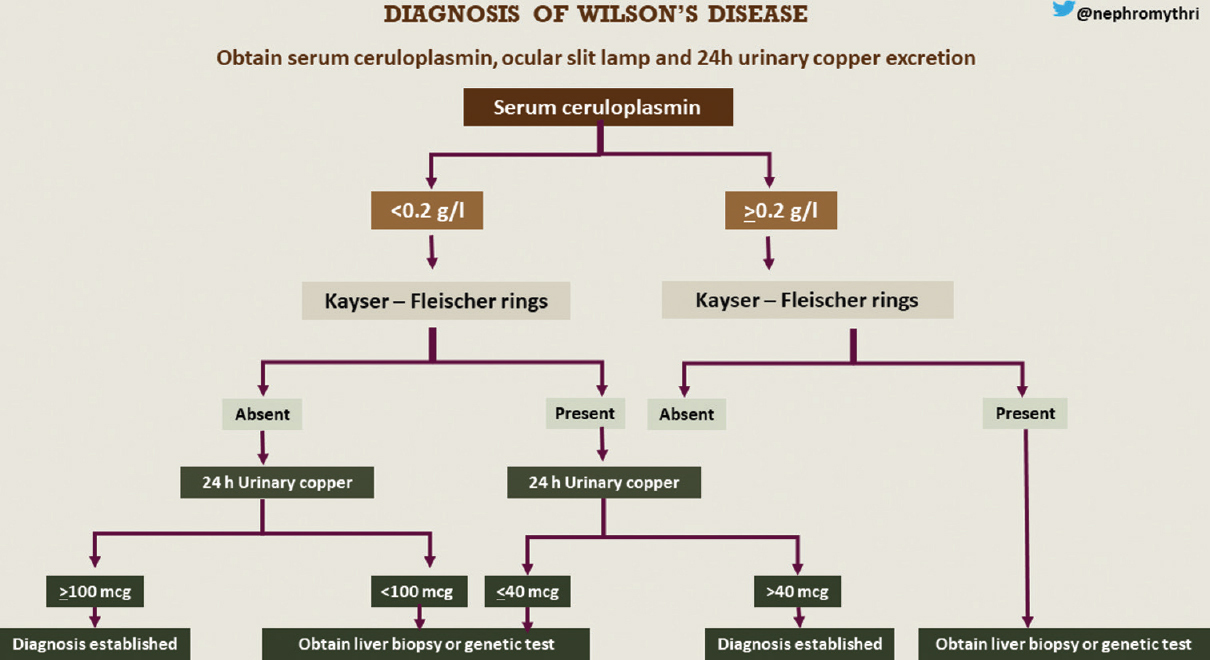
- Flowchart for the diagnosis of Wilson’s disease. Infographics credit: Mythri Shankar
Treatment
He was started on chelating agent – oral tablet D-penicillamine 500 mg/day, gradually increased to 1 g/day (500 mg twice a day), along with oral tablet pyridoxine 20 mg/day. He was also started on maintenance with oral zinc 150 mg/day given as three divided doses. He was continued on oral potassium magnesium citrate supplements for approximately 6 months. He did not require the supplements beyond that, probably due to the recovery of renal tubular epithelial cells. Four weeks following treatment, his electrolyte abnormalities had normalized. At 1-year follow-up, he did not have any recurrence of symptoms. Renal function tests and electrolytes at 1 year were as follows: s. creatinine- 1.02 mg/dl, b. urea- 22 mg/dl, s. sodium- 133 mEq/l, s. potassium- 3.7 mEq/l, s. chloride- 98 mEq/l, and s. bicarbonate- 25 mEq/l. Follow-up of urinary copper levels and s. ceruloplasmin levels was not feasible due to financial constraints. Liver function tests remained normal throughout.
Discussion
There is a wide variability in clinical presentation of Wilson’s disease with 10%–100% of patients presenting with neuropsychiatric symptoms. In this case, the diagnosis of Wilson’s disease was delayed by 4 years as the patient presented to the psychiatric department first with bipolar affective disorder. In one case report,[5] the diagnosis of Wilson’s disease was delayed by 12 years due to clinical presentation with psychiatric symptoms. This young adult presented to us with hypokalemic muscle paralysis. Hypokalemic periodic paralysis is skeletal muscle calcium channelopathy precipitated by exercise and a high carbohydrate diet.[6] It was excluded on the following grounds: 1. No precipitating factor, 2. Presence of renal tubular acidosis, and 3. Subsequently, response to penicillamine and zinc. Thyrotoxicosis-related muscle weakness was ruled out as thyroid function tests were normal.
Wilson’s disease is a rare cause of Fanconi’s syndrome. Also, distal renal tubular acidosis usually occurs due to a defect in the acidification of urine at the distal convoluted tubule (defective hydrogen ion secretion). Renal loss of sodium bicarbonate leads to hyperchloremia and hypokalemia. Also, there is increased renal loss of potassium due to excessive distal secretion of potassium. This leads to severe hypokalemia causing muscle paralysis.[7]
To our knowledge, the clinical presentation of Wilson’s disease in the form of recurrent muscle paralysis has been reported in only three cases. Chu et al.[8] reported a 24-year-old male presenting with recurrent hypokalemic muscle paralysis (due to proximal tubular acidosis) since the age of 13 years, while the diagnosis was established only at 18 years of age when he developed wing-beating tremor. It can be noted here that there was a delay in establishing the diagnosis. The age at the time of diagnosis was similar to the present case. But he developed neurological symptoms in the form of wing-beating tremors, whereas this case had a bipolar affective disorder. Also, the cause for hypokalemic muscle weakness was incomplete proximal tubular acidosis, whereas this case had both proximal and distal renal tubular acidosis. Chakraborty et al.[4] described a case of 24-year-old male with recurrent muscle weakness and the presence of KF rings, like this case. However, he had distal tubular acidosis, whereas our case had both proximal and distal tubular acidosis. Both the cases did not have hepatitis, similar to this case. Thapa et al.[9] published another case of a young male with recurrent muscle weakness and KF rings, similar to the present case. However, he had hepatitis and distal tubular acidosis alone, unlike in the present case. This is possibly the first case report of proximal renal tubular acidosis with distal tubule involvement due to Wilson’s disease.
Conclusion
Wilson’s disease may present with hypokalemic muscle paralysis due to proximal tubular acidosis with distal tubule involvement. So, in young men presenting with hypokalemic periodic paralysis with metabolic acidosis, it is apt to rule out Wilson’s disease. Also, one-third of patients with Wilson’s disease may present with psychiatric symptoms, leading to delay in diagnosis and treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Rare presentation of Wilson's disease:A case report. Int Urol Nephrol. 2004;36:289-91.
- [Google Scholar]
- Renal tubular acidosis due to Wilson's disease presenting as metabolic bone disease. BMJ Case Rep. 2011;2011:bcr0420114121.
- [Google Scholar]
- Recurrent limb weakness as initial presentation of Wilson's disease. Indian J Gastroenterol. 2007;26:36-8.
- [Google Scholar]
- Clinical presentation, diagnosis and long-term outcome of Wilson's disease:A cohort study. Gut. 2007;56:115-20.
- [Google Scholar]
- Hypokalemic periodic paralysis:A case report and review of the literature. Cases J. 2008;1:256.
- [Google Scholar]
- Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J. 2016;16:525-30.
- [Google Scholar]
- Recurrent hypokalemic muscle weakness as an initial manifestation of Wilson's disease. Nephron. 1996;73:477-9.
- [Google Scholar]







