Translate this page into:
Zebra Bodies in the Kidney: Is it a Pathognomonic Finding of Fabry Disease?
Corresponding author: Prem S. Patel, Department of Nephrology, Indira Gandhi Institute of Medical Science, Patna - 800 014, Bihar, India. E-mail: drpspdm@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patel PS, Singh PP, Krishna A, Kumar O, Archana. Zebra Bodies in the Kidney: Is it a Pathognomonic Finding of Fabry Disease? Indian J Nephrol. 2025;35:298-301. doi: 10.25259/ijn_392_23
Abstract
Zebra bodies are intralysosomal lamellar inclusion bodies. It is accepted as a specific feature of Fabry disease. However, it has been reported in many hereditary and acquired conditions. We are reporting Zebra bodies in the kidneys of cases with Rheumatoid Arthritis and hydroxychloroquine-induced phospholipidosis. Case 1: A 55-year-old male presented with hypertension and renal dysfunction. Serum ANA and anti-CCP antibodies were positive. A kidney biopsy revealed chronic tubulointerstitial nephritis with Zebra Bodies in the podocytes. Genetic analysis was negative for Fabry disease. Case 2: A 34-year-old female with Systemic Lupus Erythematosus on Hydroxychloroquine for a year presented with subnephrotic proteinuria. Serum ANA and anti-dsDNA antibodies were positive. Electron microscopy showed lamellated osmiophilic inclusion bodies in the tubular and visceral epithelial cells. Thus, Zebra bodies are not pathognomonic for Fabry disease and Rheumatoid Arthritis should also be considered in the differential diagnosis, particularly if family or drug history is negative.
Keywords
Drug-induced phospholipidosis
Fabry disease
Hydroxychloroquine
Rheumatoid arthritis
Zebra bodies
Introduction
Zebra bodies are widely accepted as a specific Fabry disease (FD) feature.1 However, zebra bodies are also reported in many hereditary diseases and drug-induced phospholipidosis.2–5 Here we describe zebra bodies in the kidneys of cases with rheumatoid arthritis and systemic lupus erythematosus.
Case Reports
Case 1
A 55-year-old male presented with hypertension and renal dysfunction for 3 months. He gave a history of pain in the small joints of both hands and wrists, associated with prolonged morning stiffness for 6 months. Joint pain was additive in nature. He did not give a history of fever, weight loss, pain in other joints, hematuria, or oliguria. He has never been evaluated and treated for joint pain. His blood pressure was 160/100 mmHg. Clinical examinations were unremarkable except for pallor, swelling, and tenderness of the proximal and distal interphalangeal joint. Laboratory values are mentioned in Table 1. Urine had bland sediments, and 24-h urine protein was 900 mg. Serum antinuclear antibody (ANA-30 U/mL) and anti-CCP antibodies (304.6 U/mL) were positive. Kidney biopsy revealed non-proliferative glomeruli, focal chronic tubulointerstitial nephritis, and interstitial fibrosis and tubular atrophy of 30%–35% of the sampled cortex. Direct immunofluorescence was negative. Electron microscopy showed enlarged visceral epithelial cells containing many osmophilic lamellated/whorl-like structures in the cytoplasm (zebra bodies) and focal foot process effacement. [Figure 1a-c] Genetic analysis (next-generation sequencing) was negative for FD. He is being treated with antihypertensive (telmisartan + hydrochlorothiazide), sodium bicarbonate, and anti-rheumatoid treatment (3 weeks tapering dose prednisolone along with sulfasalazine). Presently, arthritis is in remission and renal function is stable.
| Parameters (Normal value) | Case 1 | Case 2 |
|---|---|---|
| Total leucocyte count (mm3) | 6700 | 56,000 |
| Red blood cell count (mm3) | 283,000 | 313,000 |
| Platelet count (103/mm3) | 177 | 184 |
| Hemoglobin (g/dL) | 10.70 | 11.80 |
| ESR (mm/h) | 55 | 22 |
| Serum creatinine (mg/dL) | 2.11 | 1.89 |
| Blood urea nitrogen (mg/dL) | 52 | 28 |
| Serum sodium (mMol/L) | 129 | 137 |
| Serum potassium (mMol/L) | 5.4 | 3.8 |
| Serum uric acid (mg/dL) | 6.4 | 6.8 |
| Serum Ca2+ (mg/dL) | 8.8 | 9.2 |
| Serum phosphate (mg/dL) | 4.3 | 3.5 |
| Serum total bilirubin (mg/dL) | 1.3 | 0.9 |
| ALP (U/L) | 126 | 67 |
| SGPT (IU/L) | 25 | 33 |
| SGOT (IU/L) | 81 | 28 |
| Total serum protein (g/dL) | 6.8 | 7.2 |
| Serum albumin (g/dL) | 4.0 | 3.4 |
| RBS (mg/dL) | 118 | 123 |
| HIV/HbSAg/HCV | Nonreactive | Nonreactive |
| ANA | Positive | Positive |
| Anti-dsDNA antibodies | Negative | Positive |
| Anti-CCP antibodies | Positive | Negative |
| ANA, PR3 and MPO ANCA | Negative | Negative |
| Complement C3 (mg/dL) | 108 | 96 |
| Complement C4 (mg/dL) | 18.1 | 22 |
| USG KUB | Bilateral normal-sized kidney | Bilateral normal-sized kidney |
| Kidney histology | Chronic tubulointerstitial nephritis with the presence of zebra bodies in podocytes with focal podocyte effacement. | Lamellated osmiophilic inclusion bodies in the tubular epithelial and visceral epithelial cell cytoplasm with focal podocyte effacement. |
ESR: Erythrocyte sedimentation rate, ALP: Alkaline phosphatase, SGPT: Serum glutamic pyruvic transaminase, SGOT: Serum glutamic:oxaloacetic transaminase, RBS: random blood sugar, HIV: Human immunodeficiency virus, HbSAg: Hepatitis B surface antigen, HCV: Hepatitis C virus, ANA: Antinuclear antibody, MPO ANCA: Myeloperoxidase antineutrophil cytoplasmic antibody, PR3 ANCA: Proteinase 3 Antineutrophil cytoplasmic antibody, USG KUB: Ultrasound of the kidneys, ureters, and bladder, Anti-CCP: Anti-cyclic citrullinated peptide
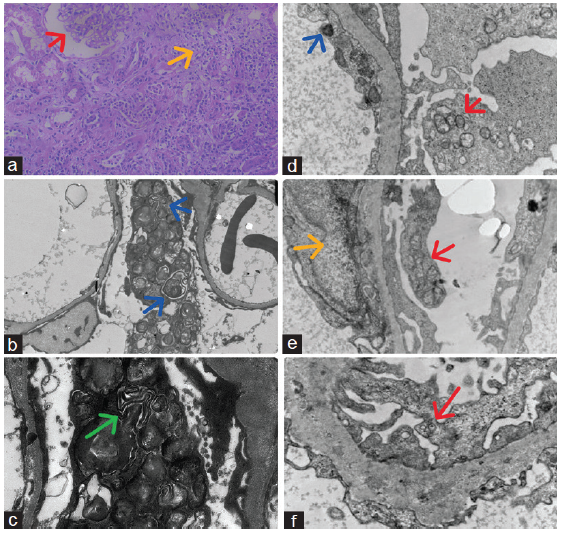
- Kidney biopsy of Case 1: (a) Shows non-proliferative glomeruli (red arrow), focal chronic tubulointerstitial nephritis (yellow arrow), and interstitial fibrosis and tubular atrophy of cortex (40×, PAS, light microscopy), (b) Show enlarged visceral epithelial cells containing many osmophilic lamellated/whorl-like structures (zebra bodies) in the cytoplasm (blue arrows) and focal foot process effacement on low magnification (1500× electron microscopy), (c) Show osmophilic lamellated/whorl-like structures (zebra bodies) in the cytoplasm of enlarged visceral epithelial cells (green arrow) and focal foot process effacement on high magnification (4000× electron microscopy), and Case 2: Electron microscopy shows (d) electron-dense deposits (blue arrow), and osmophilic lamellated/whorl-like structures (zebra bodies) in the cytoplasm of visceral epithelial cells (red arrow) (5000×), (e) tubuloreticular inclusions (yellow arrow) in endothelial cells, and osmophilic lamellated/whorl-like structures (zebra bodies) in the cytoplasm of visceral epithelial cells (red arrow) (5000×), (f) some osmophilic lamellated/whorl-like structures (zebra bodies) in the cytoplasm of visceral epithelial cells (red arrow) (8000×). PAS: Periodic acid-Schiff.
Case 2
A 34-year-old female housewife with systemic lupus erythematosus on hydroxychloroquine for 1 year presented with subnephrotic proteinuria. Clinical examination was unremarkable. Laboratory values are mentioned in Table 1. Urine had bland sediments, and 24-h urine Protein was 1500 mg. Serum antinuclear antibody (ANA- 60 U/mL) and anti-dsDNA antibodies (340 IU/mL) were positive. Serum complement levels were normal. Kidney biopsy revealed non-proliferative glomeruli without evidence of tuft necrosis, capillary wall thickening, tuft sclerosis, and subendothelial immune complex deposit. IFTA involved 8%–10% sampled cortex. Direct immunofluorescence was negative. EM showed several tubuloreticular inclusions in endothelial cells; a osmophilic lamellated/whorl-like structures in the cytoplasm of endothelial, visceral, and tubular epithelial cells; and focal foot process effacement. [Figure 1d-f] She has been advised prednisolone (0.5 mg/kg), ramipril 5 mg daily, and hydroxychloroquine.
Discussion
We describe two cases with rheumatological conditions whose kidney biopsies showed showed lamellar inclusion bodies indicative of lipid deposit. The first one was probably due to rheumatoid arthritis, and the second was presumably hydroxychloroquine-induced renal phospholipidosis. Lamellar inclusion (zebra bodies) in the kidney is widely believed as a pathognomonic for FD. However, mimics of zebra bodies have been seen in many hereditary diseases such as FD, Nail–Patella-like renal disease (NPLRD), and Niemann–Pick disease, as well as in acquired conditions such as drug-induced kidney phospholipidosis and silicon nephropathy.2,4,5 FD is characterized by a deficiency of the alpha-galactosidase-A enzyme.1,3 The lack of systemic manifestation of FD and negative genetic analysis in our first case led to the unlikelihood of FD. Due to the unavailability of tests in the institute laboratory, we did not estimate the serum or leucocyte alpha-galactosidase A level. Thus, the diagnosis of FD in the first case was excluded.
The typical histological finding in NPLRD is the curvilinear deposition of type-III collagen fibrils. Other changes include minimal-change disease, focal segmental glomerulosclerosis, foot process effacement, and normal EM.2 The absence of dysplastic nails and patella and typical basement membrane changes in the kidney of both cases excluded the possibility of LMX1B-associated nephropathy.
Drug-induced phospholipidosis refers to the intracellular lysosomal accumulation of phospholipids in the form of lamellar bodies.4 Because drug-induced phospholipidosis and FD have similar light and electron microscopic features, a diagnostic dilemma may arise, especially when the drug history is unavailable. Thus, in our second case, the history of the hydroxychloroquine treatment together with the absence of clinical manifestations and negative genetic analysis of FD established the diagnosis of hydroxychloroquine-induced renal phospholipidosis.
It is difficult to distinguish the lamellar body morphologically based on the renal cells involved and ultrastructure characteristics.6 The curvilinear inclusion bodies are highly suggestive of drug-induced phospholipidosis, but it does not seem to be uniform findings.6 Similarly, in agreement, we also did not find curvilinear bodies in either patient. These ultrastructure characteristics could be only a clue for differential diagnosis but not confirmatory features. Thus, detailed information on clinical manifestation, drug history, and genetic workup is required to establish the diagnosis. We speculate that the presence of zebra bodies in the first case was a manifestation of autoimmune activity.
Zebra bodies are non-pathognomonic. Only histological features would not help in the diagnosis. Detailed information on clinical manifestation, drug history, and genetic workup is required to establish the diagnosis. Rheumatoid arthritis should also be considered in the differential diagnosis, particularly if family or drug history is negative.
Acknowledgment
We are thankful to Dr. Alok Sharma, MD, Technical Director of Renal Pathology and Transmission Electron Microscopy, Dr. Lal Path Labs Ltd, for providing histopathology images of the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Zebra bodies in the kidney. N Engl J Med. 2020;383:e2. doi: 10.1056/NEJMicm1912889
- [CrossRef] [PubMed] [Google Scholar]
- Myelin bodies in LMX1B-associated nephropathy: Potential for misdiagnosis. Pediatr Nephrol. 2020;35:1647-57.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrastructural deposits appearing as “Zebra Bodies” in renal biopsy: fabry disease? - comparative Case Reports. BMC Nephrol. 2017;18:157. doi: 10.1186/s12882-017-0571-0
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lupus nephritis and hydroxychloroquine-associated zebra bodies: not just in fabry disease. Kidney Med. 2021;3:442-446.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal involvement in neimann-pick disease. NDT Plus. 2009;2:448-51.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and biochemical investigation of male patients exhibiting membranous cytoplasmic bodies in biopsied kidney tissues; a pitfall in diagnosis of fabry disease. J Nephropathol. 2015;4:91-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







