Translate this page into:
A Case Report of Occult Weak ‘A’ Subgroup: An Important Message for Renal Transplant Physicians
Address for correspondence: Dr. Mitu Dogra, House No. E 50, Mansarover Garden, New Delhi - 110 015, India. E-mail: drmithu25@yahoo.in
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Accurate ABO grouping is the cornerstone of a successful ABO-compatible organ transplant. While conventional methods identify blood groups accurately in most cases, rare and weak blood groups could occasionally be misread/missed. Weak A subgroups such as A3, Ax, Aend, Am, Ay, and Ael are often mistyped as group O. We present one interesting case of ‘weak A’ subgroup in a renal transplant donor, who was wrongly typed as ‘O’ Rh D positive by conventional grouping techniques. It was a near miss as the donor was almost selected for transplant for the patient with blood group B positive.
Keywords
Anti A
B antisera
blood group discrepancy
weak subgroup
Introduction
The ABO blood group system was discovered more than a century ago and still often raises uncertainty during sub-typing or detection of weaker variants. Accurate ABO grouping is the cornerstone for a successful ABO-compatible organ transplant. There are no fixed standards for how any laboratory should perform ABO blood typing. Majority of the laboratories adopt routine methods like conventional tube technique (CTT) or column agglutination techniques (CAT) using Anti-A, Anti-B and Anti-D antisera for forward typing; and A cells and B cells for reverse typing. Use of Anti-A, B antisera, Anti-A1 lectin, Anti-H lectin and A2 cells is purely optional[1] and is not used by most labs. While conventional methods identify blood groups accurately in most cases, rarer and weaker blood groups can occasionally be misread or inadvertently missed by routine testing methods. Weaker subgroups of A such as A3, Ax, Aend, Am, Ay, and Ael, due to weak reactions by conventional techniques, are often mistyped as group O and may potentially be dangerous when it comes to a solid organ transplantation.[2] Differentiation of weak A and B phenotypes require extended typing methods and specialized techniques. Anti-A, B antisera is more effective in detecting weakly expressed A and B antigens as compared to monoclonal reagents Anti-A or Anti-B.[3]
Case Report
A living donor kidney transplant was planned for a 69-year-old male with end-stage renal disease. The donor was a 50-year-old, healthy female. Both recipient and donor had undergone a preliminary pre-transplant workup at an NABL accredited pathology laboratory where blood grouping had been performed by CTT and the donor and recipient’s groups reported as O RhD-positive and B RhD-positive, respectively. Additionally, the donor also had a previous blood group report of O RhD-positive from another well-established laboratory.
As per hospital transplant workup protocol, the donor’s sample was sent to our blood bank for pre-transplant blood group confirmation. Grouping was performed using hemagglutination (HA) technique (twelve-well-typing), which included Anti-A, B antisera, Anti-A1 lectin in forward typing and A2 cells in reverse typing. Donor’s red cells were nonreactive with monoclonal Anti-A and Anti-B antisera, and Anti-A1 lectin, however, showed 2+ reaction with Anti-A, B antisera and strong agglutination reaction with Anti-H lectin (4+). On reverse grouping, the donor’s serum showed reaction with A1 cells (2+) and B cells (4+), but no reaction with A2 and O cells, indicating presence of Anti-A1 and Anti-B isoagglutinins [Table 1]. Blood grouping pattern observed on HA showed a type-II discrepancy suggestive of a weak A subgroup, which was missed by routine CTT and CAT. Blood grouping was repeated by extended CTT method, with additional Anti-A, B antisera and Anti-A1 and Anti-H lectins and A2 cells. Reaction pattern by extended CTT method showed findings similar to HA [Table 1]. To detect weak A antigen, Lui freeze–thaw elution procedure was performed on the donor’s red cells. The Lui freeze–thaw elution technique is the most simple technique and very effective for eluting ABO antibodies. This procedure is based on the principle that weak ABO subgroups are too weak to be detected by direct agglutination techniques, even after application of cold temperature and antibody enhancement. Presence of such weak A antigens, B antigens, or both can be elicited by adsorbing monoclonal anti-A or anti-B antibodies to these red cells, followed by elution of bound antibody by rapidly freezing these red cells at a temperature less than -30°C. The extracellular ice crystals that form attract water from their surroundings, increasing the osmolarity of the remaining extracellular fluid, which then extracts water from the red cells. The red cells shrink, resulting in cell lysis. As the membranes are disrupted, the isoantibodies, if adsorbed, are dissociated from the red cell surface and come into the eluate. This eluate when reacted with group specific reagent A1 or B red cells, show agglutination if any isoantibodies are present in the eluate, as the reagent red cells have adequate number of ABO antigens on their surface.[4]
| Method | Blood grouping | Adsorption-Elution | Secretory status | Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forward | Reverse | Anti A | Anti B B cells |
Anti A, B | ||||||||||||
| Anti-A | Anti-B | Anti A, B | Anti- A1 | Anti- H | A1 cells | A2 cells | B cells | O cells | A1 cells | A2 cells | A1 cells | A2 cells | ||||
| HA | 0 | 0 | 3+ | 0 | 4+ | 3+ | 0 | 4+ | 0 | 2+ | 0 | 0 | 2+ | 0 | H substance present | Ax Type |
| Extended CTT | 0 | 0 | 2+ | 0 | 4+ | 2+ | 0 | 4+ | 0 | |||||||
| CAT | 0 | 0 | N/A | N/A | N/A | 2+ | N/A | 4+ | N/A | |||||||
Key: 0=No red blood cell agglutination; 1+to 4+=Grades of red blood cell agglutination; N/A=Not applicable
The eluate showed: a) agglutination with reagent A1 cells, b) failed to agglutinate reagent O and A2cells, indicating a weak A subgroup with Anti-A1 isoantibodies, possibly indicating Aend or Ax subgroup. Serologically, Aend is almost similar to Ax and even adsorption–elution tests fail to differentiate between the two and need saliva testing for discrimination. To detect presence of soluble substances, secretory status was determined using the donor’s saliva. She was found to be a secretor, having only H substance detectable in the saliva, excluding the possibility of Aend phenotype [Figures 1a and 1b]. The serological reactions and saliva findings obtained were consistent with reactivity pattern of Ax phenotype [Table 2]. One of the donor’s children, her son, who was available on site was also tested and found to be A2B RhD-positive with anti-A1 isoagglutinins. The donor’s husband’s blood group was confirmed to be B RhD-positive. Finally, the donor was reported as Weak A subgroup RhD positive-Ax phenotype.
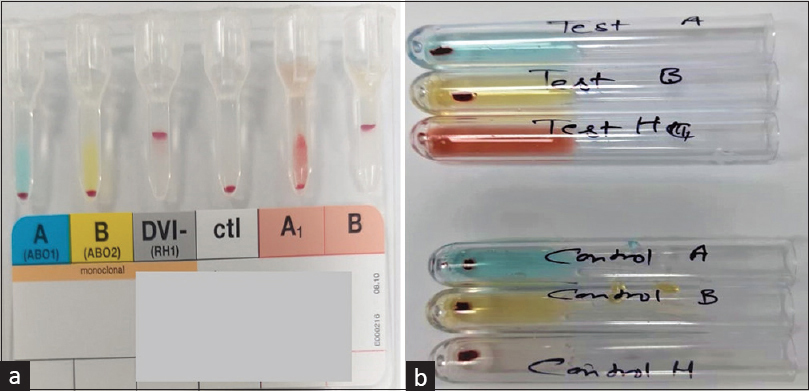
- (a and b) Part A suggests blood group of donor falsely as O-positive on column agglutination technique. Part B suggests that the donor is secreting H substance in her saliva
| Weak subgroups | Forward grouping | Reverse grouping | Substances present in saliva of secretors | Presence of A transferase in Serum | Number of Antigen sites RBC *103 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti A | Anti B | Anti-A, B | Lectin | A Cells | B Cells | O Cells | |||||
| Anti-A1 | Anti-H | ||||||||||
| A3 | ++mf | 0 | ++mf | 0 | 3+ | No | Yes | Sometimes | A, H | Sometimes | 35 |
| Ax | wk/0 | 0 | 2+ | 0 | 4+ | 0/wk | Yes | Almost always | H | Rarely | 5 |
| Aend | wk mf | 0 | wk mf | 0 | 4+ | No | Yes | Sometimes | H | no | 3.5 |
| Am | 0/wk | 0 | 0/+ | 0 | 4+ | No | Yes | No | A, H | Yes | 1 |
| Ay | 0 | 0 | 0 | 0 | 4+ | No | Yes | No | A, H | trace | 1 |
| Ael | 0 | 0 | 0 | 0 | 4+ | Some | Yes | Yes | H | no | 0.7 |
*Number of Antigen sites are important as more antigenic sites are responsible for rejection of graft
Discussion
Principles of ABO compatibility are fundamental to any solid organ transplantation. Naturally occurring IgM anti-A and/or anti-B isoantibodies present in the serum of recipient constitute a major barrier against ABO-incompatible solid organ transplantation and are overcome by adopting various desensitization protocols;[5] however, the risks of infection and rejection are higher in such transplants. Although group A2 or weaker subgroup donors have been shown to be equivalent to group O solid organ donors for non-O solid organ transplant recipients,[6] a donor of weaker subgroup, if mistyped as O group and selected as a solid organ donor for across group recipients, may lead to graft rejection due to presence of high anti-A or anti-B isoagglutinins in the recipient’s serum. Differentiating subgroup of A enables transfusion and transplant facilities to plan solid organ transplantations appropriately. In the present case study, the donor’s group was serologically identified as Ax type. The red cells of an Ax individual distinguishes themselves by giving negative or weak agglutination by monoclonal anti-A antisera, negative reactions with monoclonal anti-B antisera, but remarkably strong reactions with anti-A, B antisera. Weaker variants of A and B arise due to inheritance and expression of variant alleles at the ABO locus and are mostly identified using hemagglutination-based methods.[7] Hence, accurate ABO typing is vital for a successful organ transplantation and should be confirmed with additional studies when initial blood grouping shows a discrepancy. Detailed workup on blood groups can help identify weaker subgroups of A and B or rare Bombay and para-Bombay phenotypes so that appropriate desensitization protocols can be followed.[8]
Conclusion
To conclude, a nephrologist’s awareness of the methodology used for blood grouping of a potential solid organ donor and correct serologic interpretation of the donor’s weak A, B antigens is very important for clinical decision-making, the desensitization protocol to be used, and the patient’s well-being.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Determining ABO group of red cells and serum—tube test. In: Cohn CS, Delaney M, Johnson ST, Katz LM, eds. Technical Manual (20th ed). Bethesda, MD: AABB; 2020.
- [Google Scholar]
- The ABO blood group system. In: Harmening DM, ed. Modern Blood Banking and Transfusion Practices (3rd ed). New Delhi; Jaypee Brothers Medical Publishers (P) Ltd; 1998. p. :86-115.
- [Google Scholar]
- The Lui elution technique. A simple and efficient method for eluting ABO antibodies. Transfusion. 1985;25:433-4.
- [Google Scholar]
- Are weak blood groups important to look for in kidney transplantation?A case report on interchanging blood groups. Indian J Transplant. 2020;14:355-7.
- [Google Scholar]
- Chapter 23-ABO and H blood group system. In: Shaz BH, Hillyer CD, Roshal M, Abrams CS, eds. Transfusion Medicine and Hemostasis (2nd ed). Elsevier; 2013. p. :149-56.
- [Google Scholar]
- Detection of a rare subgroup of A phenotype while resolving ABO discrepancy. Asian J Transfus Sci. 2019;13:129-31.
- [Google Scholar]
- Serological evaluation and differentiation of subgroups of “A” and “AB” in healthy blood donor population in Eastern India.Glob J Transfus Med. . 2020;5:192-6.
- [Google Scholar]







