Translate this page into:
A prospective audit of complications in 100 consecutive pediatric percutaneous renal biopsies done under real-time ultrasound guidance
Address for correspondence: Dr. R. Sinha, 37, G Bondel Road, Kolkata - 700 019, West Bengal, India. E-mail: rajivsinha_in@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Despite being a common procedure, percutaneous renal biopsy (PRB) carries the potential for complications. The British Association of Paediatric Nephrologist (BAPN) has published standards for pediatric PRB. As Indian data are scarce, we conducted a prospective audit of 100 consecutive pediatric renal biopsies (60% males) under real-time ultrasound guidance. Nephrotic syndrome was the most common indication for PRB (68%) with minimal change disease (30%) and focal segmental glomerulosclerosis (25%) being the most common histopathological lesions. Gross hematuria was observed in six cases. Major complications was noted in one case, who needed longer hospital stay. The result of the audit demonstrated achievability of BAPN standards. In addition, we also show the usefulness of 16 gauge biopsy needle over 18 gauge biopsy needles (median number of glomeruli 25, range 3–90 vs 13, range 6–46, P = 0.001) without any increase in complications. Being a single center study, we do hope that our results will encourage a wider survey on the current state of pediatric PRB.
Keywords
Audit
biopsies
complications
pediatric
renal
Introduction
Introduction of safe renal biopsy has been hailed as one of the important paradigm shift in nephrology and contributed significantly to the establishment of nephrology as a separate discipline.[1] Iversen and Brun get the credit for publishing the first series of percutaneous renal biopsy (PRB) in 1951, which was done in sitting position and, thereafter, Kark and Muehrcke published their experience in 1954 using prone position, the current standard procedure.[23] Since then, there has been steady improvement in the technique of PRB including real-time ultrasound (USG) guidance and automated biopsy needles. Despite this, it still carries significant potentials for complications, partly due to lack of standardization of practice.[45] Keeping this in mind, The British Association for Paediatric Nephrology (BAPN) published a minimal standard guideline for assessing the performance of pediatric PRB.[6] Indian data are either retrospective or have small sample size [Table 1].[789] One of the ways quality can be assessed is through clinical audit, and comparison against a set standard.[10] In this manuscript, we report prospective audit of 100 consecutive pediatric PRB assessed against BAPN criteria.[6] To the best of our knowledge, this is the largest such prospective data series from India.

Materials and Methods
A prospective audit of 100 consecutive pediatric renal biopsies was undertaken at the Vision Care Hospital, AMRI, Kolkata between July 2012 and September 2014. All biopsies were done after admission. Verbal information about the procedure and its complications were given to all parents, and a standardized informed written consent obtained prior to the procedure. Standard investigations included complete hemogram, coagulation profile, viral (hepatitis B, hepatitis C, and human immunodeficiency virus) serology and blood group testing. Abnormal hemostasis, uncontrolled hypertension, and active urinary tract infection were ruled. In the presence of coagulation abnormalities, results were repeated after Vitamin K injection, and if still abnormal, biopsies were done under the cover of fresh frozen plasma. No biopsies were undertaken in the presence of disseminated intravascular coagulopathy or active bleeding. Children were temporarily shifted to Pediatric Intensive Care Unit, and biopsies done at the bedside in the presence of persons skilled in pediatric advanced life support.
The procedure was done under deep sedation with intravenous midazolam and ketamine.[11] Children with blood pressure ≥90th centile for age and sex were given a combination of fentanyl and propofol to prevent worsening hypertension through catecholaminergic effects of ketamine.[12] Heart rate, blood pressure, electrocardiography, respiratory rate, and oxygen saturation were monitored during the procedure and until full recovery from anesthetics. Supplemental oxygen was given as necessary. Equipment and drugs for resuscitation and emergency airway support including intubation were kept ready for all children.
All renal biopsies were performed under real-time USG guidance wherein both visualization of kidney and biopsy [Figure 1] were done by a single operator. Native biopsies were done in a prone position and transplant in supine. In cases of native biopsies, the lower pole of the left kidney was targeted with automated biopsy needle (Bard®). Two cores were attempted for light microscopy and immunofluorescence whereas an additional core was taken if electron microscopy was deemed necessary. USG was done immediately post PRB to identify any peri-renal hematoma.

- Real time ultrasound – guided percutaneous renal biopsy by a single operator
Post biopsy vitals were monitored every 15 min for the 1st h, every 30 min for next hour, hourly for next 2 h, 2 hourly for another 2 h, and thereafter 4 hourly. Urine was collected, and the sample stored in the separate container to monitor gross hematuria if any. If the child was hemodynamically stable s/he was transferred to the pediatric ward after 6 h, and discharged the next day if stable. In the presence of gross hematuria or significant hemodynamic instability, a repeat USG with Doppler was done within 24 h. Paracetamol was given round the clock (4 hourly) for 48 h. If pain was not controlled adequately with this, codeine was prescribed as an additional analgesic.
An audit proforma was used wherein demographic details, indications for biopsy, biochemical (creatinine, albumin)/hematological (blood count and coagulation profile)/serological parameters, type of sedation, number of attempts (passes), number of cores, biopsy needle size, and any complications post renal biopsy were noted. Final audit was done against the BAPN audit criteria [Table 2].

Descriptive statistics was used to define baseline variables. Test for significance was done by Chi-square for nominal data and ANOVA for parametric/nonparametric data. P < 0.05 was considered as significant. Statistical analysis was performed using Smith's Statistical Package (SSPVersion 2.80, September 26, 2011, Manufacturer Gary Smith, UK).
Results
Demographic analysis of our 100 cases showed a male preponderance (60%) with a median age of 8 years (range: 0.3–17 years). Ketamine and midazolam were used for sedation for 62% of children and propofol and fentanyl for the rest. There was no sedation related complications.
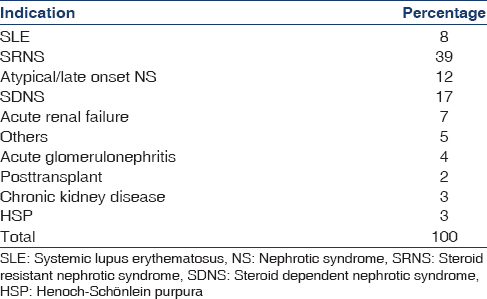
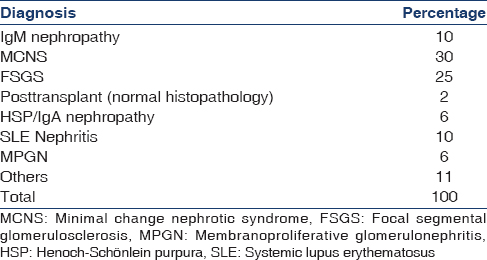
The clinical indications for biopsy for native kidneys (n = 98) are shown in Table 3 and the final histopathological diagnosis in Table 4. The criteria for biopsy of transplant kidneys (n = 2) was at least 10% rise in plasma creatinine from baseline or other clinical indication of possible rejections or recurrence. The indications for biopsy in native kidney steroid resistant nephrotic syndrome (NS) i.e., SRNS = 39%, steroid dependent nephrotic syndrome prior to starting calcineurin inhibitor or rituximab = 17%, and atypical/late onset NS = 12%. The most common histopathological diagnoses were minimal change NS (30%), focal segmental glomerulosclerosis (25%), systemic lupus erythematosus nephritis (10%), and IgM nephropathy (10%).
The biopsy was done by 16 gauge needles in 71% cases and 18 gauge needles in 28% cases. One 3 months old had a biopsy with 20 gauge needle. Two passes gave adequate kidney tissue in 72% of cases whereas another 25% children required total 3 passes. The median pass/attempt to core ratio was 1 (range 1–2). Histopathologist found the tissue to be satisfactory for 100% samples for light microscopy and in 99% samples for immunofluorescence. The median number of glomeruli per biopsy was 22 (range 3–76). In 56% cases, the specimen had ≥20 glomeruli, and 22% had 10–19 glomeruli. Only 2 children had less than five glomeruli. About 6% of the children had gross hematuria. Apart from one, all resolved within 8 h, and none required blood transfusion or any intervention. Immediate post renal biopsy USG did not reveal any significant hematoma (>2 cm). The median number of glomeruli obtained from biopsy with 16 gauge (median 25, range 3–90) was significantly greater than those obtained by18 gauge needle (median 13, range 6–46), P = 0.001. Gross hematuria happened was numertically more frequent with 18 than 16 gauge needle (n = 2, 7.1% vs n = 4, 5.6%). Gross hematuria correlated with attempts to core ratio (1.5 ± 0.44 vs. 1.1 ± 0.26, P = 0.002). We did not find any correlation between gross hematuria and sex or age. The audit result as per BAPN criteria is shown in Table 2.
Discussion
Kidney being a highly vascular organ, PRB (although a relatively safe procedure) does have the potential of resulting in complications.[13] Hence, although the likelihood of getting an adequate tissue is as high as 99–100%,[914] its success is not measured only by the adequacy of the biopsy tissue but also by the safety of the procedure. Biopsy is more challenging in children because of various factors including wide range of size, variable level of patient cooperation, and variable degree of analgesia/anesthesia requirement.[4] Despite being a common procedure in pediatric nephrology, recent surveys have shown lack of uniformity of practice even in developed countries and data from India remains scanty.[45789] In this current largest prospective audit on pediatric PRB from India performed under real-time USG guidance by a pediatric nephrologist, we have demonstrated its overall efficacy as well as safety to be at par with BAPN standards.
All our biopsies were done with full resuscitation per the European Society of Paediatric Radiology (ESPR) guidelines.[15] USG guidance was used because of advantages such as continuous visualization of both kidney and needle, avoiding exposure to radiation, permitting procedure to be performed at bedside and avoiding administration of nephrotoxic contrast media.[16] Recent studies have demonstrated the advantage of real time USG guidance,[17] as well as safety and efficacy of nephrologist, performed USG guided PRB over radiologist performed PRB.[18]
Similar to previous Indian studies,[789] various forms of NS were the most common indications for the biopsies [Table 3]. In contrast to the BAPN survey[4] where in Henoch Schonlein Purpura nephritis was the most common final histopathological diagnosis, we found both minimal change nephrotic syndrome and focal segmental glomerulosclerosis [Table 4] to be more common probably reflecting the known higher incidence of NS among the Asian population.[1920]
Unlike the studies by either Mahajan et al.[8] or Chopra et al.,[9] most of the biopsies were performed with 16 gauge needles (71%). Whereas this is in contrast to the ESPR recommendation,[15] this reflects the general trend among the nephrologist to use bigger needles.[132122] Similar to the large Norwegian registry analysis, we found that 16 gauge needles yielded statistically greater numbers of glomeruli with a non significant trend of higher complications.[23] This apparently paradoxical increase in complications has been postulated to be due to the tendency of thinner needles to deviate from the lower pole to proximal direction (where vessel density is higher), with subsequent bleeding into the pelvis.[23] Only a single previous Indian study[9] documented average yield and compared to that our yield of glomeruli (median 22 vs. 17) was higher. We recorded ≤2 attempt/pass in 72% cases whereas the Norwegian study[23] reported adequate biopsy material from 85%. We postulate that our slightly lower percentage might be due to the absence of dissecting microscope in our procedure room wherein we were often forced to take an additional sample to ensure that we have got adequate number of glomeruli.
The success of PRB should be determined both by the adequacy of the tissue and the incidence of complications. Gross or macroscopic hematuria continues to be the most frequent important complication if it necessitates blood transfusion, surgical intervention/embolization or prolonged hospital stay.[4232425262728] In our series, gross hematuria occurred in 6%, lower than 49.6% observed by Nammalwar et al.,[7] it was similar to the other Indian reports.[89] Among the pediatric studies from India, Chopra et al.[9] also reported similar findings, but in the report from Mahajan et al.,[8]3% developed hemodynamic compromise and needed a blood transfusion. In the Norwegian study of 715 children,[23] one needed transfusion and another embolization/surgical intervention. Post-biopsy hematomas[26] greater than 2 cm is likely to have clinical significance,[29] and we did not document any such. Nammalwar et al.[7] documented subcapsular hematoma in 6% cases, Mahajan et al.[8] in 7% and Chopra et al.[9] in none, whereas the Norwegian study reported an incidence of 8.1%.[23] In contrast, there are studies documenting higher incidence of perirenal hematomas ranging from 30% to 90%.[2630] This discrepancy is likely to be a reflection of how extensively they were looked for and as to whether there were any size limits of hematomas for documentation. AVF is another potential complication of PRB, which are often silent. Angiography-based imaging studies have reported an incidence of about 10% with the majority (over 90%) resolving spontaneously a year later. Significant AVF usually presents with hypotension, and although none of our children experienced significant hypotension, we did look for AVF among those with gross hemorrhage but none was found. Among the Indian studies significant hypotension were noted by both Nammalwar et al. as well as Mahajan et al., but none of them mentioned AVF.[78] The Norwegian study reported a single AVF in their series of 9288 biopsies in both adults and children.[23] Catastrophic complications such as renal loss or loss of life was not seen in our study and neither was it reported in either of the recent Indian reports.[89] Nammalwar et al. did report the loss of a kidney, but it analyzed a period ranging from 1998 to 2002 through which period the procedure had evolved from blind renal biopsy to USG guided.[7] Fortunately, the introduction of USG guidance and the automated biopsy gun has reduced the rate of catastrophic complications to practically nil as evident in more recent publications.[489142324] Studies have tried to identify risk factors for post renal biopsy bleeding with variable results. In the prospective assessment by Manno et al.[26] among 471 adult patients, younger age, female sex, and prolonged partial thromboplastin time were found to be significant predictors after adjusting for confounding factors. On the other hand in the Norwegian study,[23] chronic kidney disease stages 3–5 and smaller center size (<30 biopsies/year) were the significant risk factors. Although we did not found any correlation between ages, sex, or creatinine value with the risk of gross hematuria, similar to Ori et al.[25] we did observe a significant link between number of attempts and gross hematuria.
Analyzing our performance as per BAPN criteria [Table 2],[6] we met most of them but also identified certain issues that could be improved.
All patients should receive appropriate written information about the biopsy procedure
None of the Indian studies mentioned of providing written information. Even the UK audit[4] found that 6 of the 11 centers did not provide families an information sheet or booklet about the renal biopsy. Although we did discuss the biopsy indication and complications with the parents, we also did not provide any written information (post-audit this is currently under production).
For both native and transplant biopsies ≤3 passes should be achieved in 80% of occasions
We were able to achieve this in 97%. Whereas neither Nammalwar et al.[7] nor Suri et al.[8] documented number of passes, Chopra et al.[9] achieved a 100% whereas in BAPN audit[4]3 out of 11 centers could not achieve this target and scored below 80%.
There should be adequate tissue for diagnosis on 95% of occasions
Adequacy has been defined by BAPN as to histopathologist being able to reach a diagnosis based on the sampled tissue. Using this criterion, Chopra et al.[9] reported a 100% success whereas keeping a cut-off of 10 glomeruli they were able to achieve the target in 96.5% cases. In the UK audit,[4]97.5% of tissue sample were adequate for light microscopy and 80.5% for immunofluorescence. We achieved 100% adequacy for light microscopy and 99% for immunofluorescence, whereas ≥10 glomeruli were obtained in 78% cases.
Major complication should be <5%
BAPN defined major complication as delay in patient discharge as a result of postbiopsy complications or requirements for further investigations, interventions, or monitoring as a result of a biopsy. The UK audit[4] found major complications in 10.4% wherein only 3 out of the 11 centers studied achieved the target of <5%. Unlike the original BAPN standard, Chopra et al.[9] defined major complications as all cases of gross hemorrhage and quoted a rate of 3.5% but did state that none required further intervention. Similarly, although we found gross hemorrhage in 6% cases, our major complication rate was only 1% (since only a single child required prolonged monitoring necessitating extension of hospital stay).
Despite the obvious strength of being the first Indian paper to prospectively analyze the outcome of pediatric PRB through a systematic audit in 100 consecutive children, our paper does have some major limitations. Being a single center experience, the results will be difficult to extrapolate to mirror the state in the country. Second, although it is the largest number of pediatric PRB prospectively analyzed from India, it still is a relatively small number, and some of our conclusions are likely to be influenced by this small numbers. These limitations have to be interpreted in the light of the objective of this audit which was exclusive to compare our current practice of PRB with a set standard i.e., BAPN.[6]
Conclusion
We demonstrated that BAPN standards[6] in pediatric PRB are achievable even in Indian setup. Simultaneously, we identified some constraints including the need for a proper information leaflet for parents, so that the family and the child can be properly prepared for this process and need for dissecting microscope in the procedure room so that if satisfactory tissue has been obtained further attempts/passes can be avoided. Last, it is envisaged that this audit will lead to a pan-India study of the current state of pediatric PRB because as shown by the UK/French publications,[45] there can be wide variations between centers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The introduction of renal biopsy into nephrology from 1901 to 1961: A paradigm of the forming of nephrology by technology. Am J Nephrol. 1997;17:347-58.
- [Google Scholar]
- British Association of Paediatric Nephrology. Renal biopsies in children: Current practice and audit of outcomes. Nephrol Dial Transplant. 2010;25:485-9.
- [Google Scholar]
- Renal biopsy practice in France: Results of a nationwide study. Nephrol Dial Transplant. 2010;25:3579-85.
- [Google Scholar]
- Experience of renal biopsy in children with nephrotic syndrome. Pediatr Nephrol. 2006;21:286-8.
- [Google Scholar]
- Should ultrasound guided percutaneous renal biopsy in children be done in a day care setting? Indian J Nephrol. 2010;20:21-4.
- [Google Scholar]
- Percutaneous renal biopsy in children: Are British association of pediatric nephrology standards achievable? Indian J Nephrol. 2014;24:130-1.
- [Google Scholar]
- Efficacy and safety of intravenous midazolam and ketamine as sedation for therapeutic and diagnostic procedures in children. Pediatrics. 1997;99:427-31.
- [Google Scholar]
- Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology. 2005;103:20-4.
- [Google Scholar]
- Nephrology and the percutaneous renal biopsy: A procedure in jeopardy of being lost along the way. Clin J Am Soc Nephrol. 2012;7:1545-7.
- [Google Scholar]
- Ultrasound-guided percutaneous renal biopsy in 295 children and adolescents: Role of ultrasound and analysis of complications. PLoS One. 2014;9:e114737.
- [Google Scholar]
- ESPR uroradiology task force and ESUR Paediatric Work Group – Imaging recommendations in paediatric uroradiology, part VI: Childhood renal biopsy and imaging of neonatal and infant genital tract. Minutes from the task force session at the annual ESPR Meeting 2012 in Athens on childhood renal biopsy and imaging neonatal genitalia. Pediatr Radiol. 2014;44:496-502.
- [Google Scholar]
- Percutaneous renal biopsy: Comparison of blind and real-time ultrasound-guided technique. Semin Dial. 2007;20:355-8.
- [Google Scholar]
- Percutaneous real-time ultrasound-guided renal biopsy performed solely by nephrologists: A case series. Indian J Nephrol. 2010;20:137-41.
- [Google Scholar]
- Disease course in steroid sensitive nephrotic syndrome. Indian Pediatr. 2012;49:881-7.
- [Google Scholar]
- High incidence of minimal change nephrotic syndrome in Asians. Arch Dis Child. 1985;60:1018-20.
- [Google Scholar]
- Native renal biopsies: Complications and glomerular yield between radiologists and nephrologists. J Nephrol. 2005;18:553-8.
- [Google Scholar]
- A prospective randomized trial of three different sizes of core-cutting needle for renal transplant biopsy. Kidney Int. 2000;58:390-5.
- [Google Scholar]
- Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7:1591-7.
- [Google Scholar]
- Standards for renal biopsies: Comparison of inpatient and day care procedures. Pediatr Nephrol. 2003;18:53-6.
- [Google Scholar]
- Using the automated biopsy gun with real-time ultrasound for native renal biopsy. Isr Med Assoc J. 2002;4:698-701.
- [Google Scholar]
- Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004;66:1570-7.
- [Google Scholar]
- Safety profile of paediatric percutaneous ultrasonography-guided renal biopsies. Singapore Med J. 2010;51:481-3.
- [Google Scholar]
- Percutaneous kidney biopsies. Complications and their management. Urology. 1981;18:118-22.
- [Google Scholar]
- Ultrasonography as a predictor of overt bleeding after renal biopsy. Clin Exp Nephrol. 2009;13:325-31.
- [Google Scholar]
- Ultrasonography and computed axial tomography in the detection and monitoring of renal hematomas following ultrasonically guided percutaneous renal biopsy. Ann Radiol (Paris). 1985;28:25-7.
- [Google Scholar]







