Translate this page into:
A Randomized Controlled Trial Comparing Remission Induction with Modified Multitarget Therapy with Intravenous Cyclophosphamide in Proliferative Lupus Nephritis
Address for correspondence: Prof. Arpita Ray Chaudhury, 244 AJC Bose Road, Kolkata - 700 020, West Bengal, India. E-mail: lahiri.arpi@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Therapy of proliferative lupus nephritis (PLN) is yet to be optimized. Standard of care for induction consists of intravenous (IV) cyclophosphamide (CYC) and steroids, which shows an improved outcome, but end-stage renal disease (ESRD) progression, increased mortality, and therapy-related adverse effects remain a major concern. The other treatment reported to induce early remission was the multitarget therapy comprising tacrolimus, mycophenolate, and steroid, but infections were high in the multitarget therapy. Considering azathioprine as a potentially safer and effective alternative anti-B-cell therapy, modified multitarget therapy (MMTT) was planned replacing mycophenolate with azathioprine.
Material and Methods:
A single-center, 24-week, open-label, randomized controlled trial comprising adults of age 18–65 years with biopsy-proven PLN was carried out. The intervention groups were 1) MMTT: tacrolimus 0.075 mg/kg/day and azathioprine 2 mg/kg/day and 2) IV CYC group with a starting dose of 0.75 (adjusted to 0.5–1.0) g/m2 every 4 weeks for 6 months. Both groups received 3 days of pulse methylprednisolone followed by a tapering course of oral prednisone therapy.
Results:
Among 100 randomized patients, 48 were in MMTT arm and 52 were in IV CYC arm. At the end of 24 weeks, overall remission (complete and partial) was comparable in both the arms: MMTT (86.36%) and IV CYC (87.75%). There was comparable proteinuria reduction and systemic lupus erythematosus disease activity index (SLEDAI) score improvement with recovery of complement level C3 in both groups. Major adverse events were numerically more in the IV CYC group, including one death from pneumonia.
Conclusion:
The MMTT arm is as effective as IV CYC in improving short-term outcome in PLN, with a comparable safety profile.
Keywords
Azathioprine
cyclophosphamide
modified multitarget therapy
proliferative lupus nephritis
randomized controlled trial
Introduction
In spite of several attempts to improve long-term outcome, proliferative lupus nephritis (PLN) still continues to be a big therapeutic challenge. Around 30%–40% patients of lupus nephritis (LN) progress to end-stage renal disease (ESRD) at the end of 10–15 years.[12] Standard of care therapy based on glucocorticoids and cyclophosphamide (CYC) had definitely improved the dismal prognosis of earlier days,[34] but short-term renal response is still limited to the rate of 44%–54% with the standard induction regimen[56] and progression to ESRD is not improving. In an older study from our institute in PLN treated with either the NIH protocol or mycophenolate mofetil revealed an outcome of only 44% complete remission (CR) and partial remission (PR) at the end of 6 months and around 64% at the end of 1 year.[7] CYC or mycophenolate mofetyl (MMF) and steroid-based induction therapy also increase the risk of many untoward side effects like sepsis, amenorrhea, hemorrhagic cystitis, and malignancy.
Pathogenesis of LN involves immune activation of B cells, T helper cells, and multiple cytokine release. Therefore, it was hypothesized that patients may benefit from a multipronged attack with three different classes of drugs.[8] Multitarget therapy may allow better response early at a lower dose of each drug, thus minimizing the side effects. Achieving early remission, CR or PR, is associated with better long-term outcome.[6] One study showed triple drug therapy comprising tacrolimus, MMF, and steroids as remission induction therapy to show highest short-term remission rate.[9] But adverse events (AEs), particularly infection related, reported in trial were quite high (50.3%), and infection-related AEs (28.2%) accounted for high dropout from the triple drug arm. We modified the triple drug therapy using tacrolimus, azathioprine, and steroids and reported a pilot study comparing Tac–Aza–Pred with standard of care as induction with follow-up for 6 months.[10] Herewith, we are reporting this randomized controlled trial to compare the safety and effectiveness of the modified triple drug regimen, comprising tacrolimus, azathioprine, and steroids (modified multitarget therapy [MMTT]), in one arm and intravenous (IV) CYC and steroids in the other arm in the management of PLN.
Materials and Methods
Setting and participants
This single-center, prospective, open-label, randomized, parallel-group, interventional study was carried out in the Department of Nephrology and Rheumatology of a teaching tertiary care hospital in eastern India. The trial compared a modified triple drug induction therapy comprising tacrolimus, azathioprine, and steroid in PLN patients with IV CYC and steroid induction. As treatment modalities comprised an IV CYC and other oral drug regimen, we conducted an open-label study for logistic constraints. The study was approved by the institutional ethics committee and registered under CTRI (CTRI2017/05/008556).
The study included patients, male or female, 18–65 years of age, either in active disease, treatment naïve, or in relapse, without any immunosuppression for the last 6 months, with biopsy-proven PLN of class III, IV, and either III or IV mixed with V (as per the ISN-RPS 2003 classification), while pure class V was excluded. Lack of eligibility criteria is shown in the trial flow [Figure 1]. All enrolled participants were followed up prospectively for 6 months.

- Trial flow: Randomization with inclusion and exclusion criteria and analysis at 6 months, CYC = cyclophosphamide, eGFR = estimated glomerular filtration rate, IV = intravenous, MMTT = modified multitarget therapy
Randomization and intervention
Randomization was done by computer-generated random number list with 1:1 allocation ratio, which was concealed by sequentially numbered sealed opaque envelopes. The study spanned over a period of 2 years from 2016 to 2018. All enrolled participants were followed up prospectively for 6 months. The study arms were the following.
Standard of care arm: (IV CYC and steroid) Methylprednisolone 500 mg IV daily for 3 days followed by IV CYC 0.75 g/m2 monthly (increased up to 1 g if tolerated) for 6 months plus oral prednisolone 0.5 mg/kg/day and gradually tapered to 7.5 mg/day over 3 months.
MMTT arm: (MMTT) Methylprednisolone 500 mg IV daily for 3 days followed by oral tacrolimus (0.075 mg/kg/day) plus azathioprine (2 mg/kg/day) plus oral prednisolone 0.5 mg/kg/day tapered to minimum dose of 7.5 mg over 3 months, with the total duration of this induction phase being 6 months. (Target trough tacrolimus 5–10 ng/ml).
Supportive therapy was similar in each group and included hydroxychloroquine (6.5 mg/kg), antihypertensives as needed, cotrimoxazole prophylaxis, and osteoporosis prophylaxis (calcium and vitamin D tablets). In the maintenance phase, tapering of tacrolimus was continued with a lower target tacrolimus level and the patients were continued on tacrolimus with a plan to stop tacrolimus by 1 year, when the patients would continue on azathioprine and steroid in both the arms. Relapse, if any, would be treated with introduction of MMF as fresh induction.
Follow up
Systemic lupus erythematosus disease activity index (SLEDAI) score was assessed and blood biochemistry like fasting blood sugar (FBS) and lipid profile, serum albumin, calcium, phosphate, alkaline phosphatase, and uric acid was done at the initiation of therapy, at 3 and 6 months and thereafter every 6 months. Complete hemogram was repeated on day 10–14 of CYC pulse and for both groups at 1, 3, and 6 months, as well as when required. Urine analysis at one-monthly interval till remission was done, and then at two-monthly intervals till 6 months and at three-monthly intervals till the end of the study. Trough tacrolimus levels were measured in arm II, on day 3 of starting tacrolimus, then at day 7, and then repeated weekly till therapeutic level of 5–10 ng/ml was attained, and thereafter if warranted by the clinical features of tacrolimus toxicity or increasing serum creatinine levels and the dose was adjusted accordingly.
Outcome
Primary outcome: Overall remission (CR + PR)
-
1)
CR was defined as return of serum creatinine to baseline or stabilization of serum creatinine ≤30% of baseline and decline in 24-h urinary protein <500 mg and disappearance of active urinary sediment (defined as urine ≥5 red blood cells per high-power field in urine sample microscopy).
-
2)
PR was defined by proteinuria <2 g/day or 24-h protein <50% of baseline but more than 500 mg/day, with stabilized serum creatinine within 1.2 mg/dl and absent urinary sediment.
Secondary outcome
-
1)
Renal relapse: Relapse was defined as an increase in serum creatinine by ≥30% above baseline or absolute increase in proteinuria >500 mg/g.
-
2)
Improvement in SLEDAI score.
Treatment-emergent AEs during the study period were captured and reported, either major or minor. Major AEs were listed as those which led to death and hospitalization and were causally related to study drugs, and all others were enlisted as minor AEs [Table 1].
| Category of adverse event | MMTT arm (n=48) | Cyclophosphamide arm (n=52) | P |
|---|---|---|---|
| Major, n (%) [95% CI] | 5/48 (10.41%) [1.08-10.24] | 8/52 (15.38%) [2.20-13.05] | 0.56 |
| Pneumonia - 2 | Pneumonia - 6 (including one death) | ||
| Soft tissue abscess - 1 | Posterior reversible encephalopathy | ||
| Cytopenia - 2 | syndrome - 2 | ||
| Minor, n (%) [95% CI] | 10/48 (20.83%) [0.61-8.76] | 13/52 (25%) [0.61-8.76] | 0.64 |
| Vomiting - 2 | Scabies - 1 | ||
| Superficial fungal infection - 1 | Upper RTI - 4 | ||
| Urinary tract infection - 1 | Herpes zoster - 1 | ||
| New-onset hypertension - 6 | Vomiting - 4 | ||
| Abnormal LFT - 2 | |||
| Hyperglycemia - 1 |
CI=Confidence interval, MMTT=Modified multitarget therapy, RTI=Respiratory tract infection
Statistical analysis
Sample size calculation was done with the primary endpoint, that is, overall remission rate (CR + PR). Assuming a CR and PR of 83% in the control arm 9 an effect size of 25%, alpha of 0.05, and 80% power with 1:1 allocation ratio, we would need 98 evaluable subjects, that is, 49 in each arm. We described categorical variables as number and percentages. We used mean (standard deviation) for normally distributed continuous variables and median (interquartile range) for non-normal continuous variables. Categorical outcomes were compared using Chi-square or Fisher’s exact test as appropriate. Normally distributed outcomes were compared using independent sample t-test. We used Mann–Whitney U test to compare data which were non-normally distributed. Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) statistical software version 20.
Results
Two hundred and five patients were screened, of whom 62 were excluded for not satisfying the inclusion criteria and 43 patients declined to give consent due to various reasons (commonest being not willing to accept new therapy). One hundred patients were randomized, with 48 patients in the triple drug arm; two patients were lost to follow up and two patients were excluded as they had conceived in between. Fifty-two patients were randomized to the IV CYC arm, of whom three were lost to follow-up. Baseline data [Table 2] for both the groups at recruitment was comparable, except that 12 patients (25%) in the triple drug group and 24 patients (46.2%) in the IV CYC group (P < 0.03) had hypertension and serum creatinine at baseline was 0.90 (0.36) in the MMTT arm versus 1.1 (0.51) in the IV CYC arm (P < 0.03). The mean values of serum creatinine were within acceptable normal limits though they were statistically different. Representation from various histopathologic classes of lupus nephritis was similar in both the arms.
| MMTT arm (n=48) | SOC arm (n=52) | P | |
|---|---|---|---|
| Age | 28 (9.5) | 28.3 (8.7) | 0.87 |
| Gender | |||
| M/F | 5/48 | 5/52 | 0.99 |
| SLE class | |||
| Class 3 | 5 (10.4%) | 6 (11.5%) | 0.90 |
| Class 3+5 | 5 (10.4%) | 6 (11.5%) | |
| Class 4 | 31 (64.6%) | 30 57.7%) | |
| Class 4+5 | 7 (14.6%) | 10 (19.2%) | |
| Hypertension | |||
| Present | 12 (25%) | 24 (46.2%) | 0.03 |
| SLEDAI | 17.7 (6.5) | 19.0 (7.4) | 0.35 |
| Serum creatinine (mg/dl) | 0.90 (0.36) | 1.1 (0.51) | 0.03 |
| Complement C3 (mg/dl), mean (SD) | 58.8 (27.33) | 59.4 (32.35) | 0.92 |
| Complement C4 (mg/dl), mean (SD) | 13.7 (7.06) | 14.1 (8.47) | 0.80 |
| 24-h urine protein (g/day) | |||
| Median (interquartile range) | 2.55 (1.58-3.56) | 2.15 (1.39-4.09) | 0.96 |
| Active urine sediment | 39 (81.3%) | 40 (76.9%) | 0.60 |
| Activity index | 5.43±2.22 | 5.46±2.0 | 0.94 |
| Chronicity index | 2.14±1.58 | 9 2.52±1.54 | 0.22 |
MMTT=Modified multitarget therapy, SD=Standard deviation, SLEDAI=Systemic lupus erythematosus disease activity index
In the control group in which 14 patients who received prior immunosuppression, the time gap between biopsy and therapy was more than a year. The remaining 38 patients were treatment naïve and received immunosuppression within 2–4 weeks of biopsy. In the MMTT group, 17 patients received prior immunosuppression. The remaining 31 patients were biopsied and they received therapy as naïve patients, with a similar time gap of 2–4 weeks. Among these 17 patients, 12 had IV CYC induction, followed by azathioprine maintenance in eight, and maintenance with MMF in four patients or more than a year, followed by noncompliance for at least 6 months and then enrolled in the MMTT group. The remaining 31 in the MMTT group were treatment naïve.
No patient in both the arms had crescentic lupus nephritis (>50% glomeruli having crescents), two patients in the SOC group had 25% and five patients had 10% crescents, and 45 patients did not have any crescent. The MMTT arm had 25% crescents in four patients and 10% crescents in three patients. We did not include pure class V patients and excluded patients with biopsy-proven thrombotic microangiopathy (TMA) and patients with high chronicity score. Average activity and chronicity indices in the SOC group and MMTT group were comparable [Table 2].
Effectiveness
No patients required dialysis in any arm. Twenty-six patients in the SOC arm and 20 patients in the MMTT arm had received renin angiotensin aldosterone System (RAAS) blockade.
Each arm showed significant improvement in SLEDAI score from baseline to M3 and M6. The mean reduction of SLEDAI score in the two arms was similar at 3 and 6 months [Figure 2a]. There was significant improvement in 24-h urine protein excretion [Figure 2b], serum creatinine [Figure 2c], and C3 level recovery [Figure 2d] in both the arms from baseline to the end of third and sixth months.
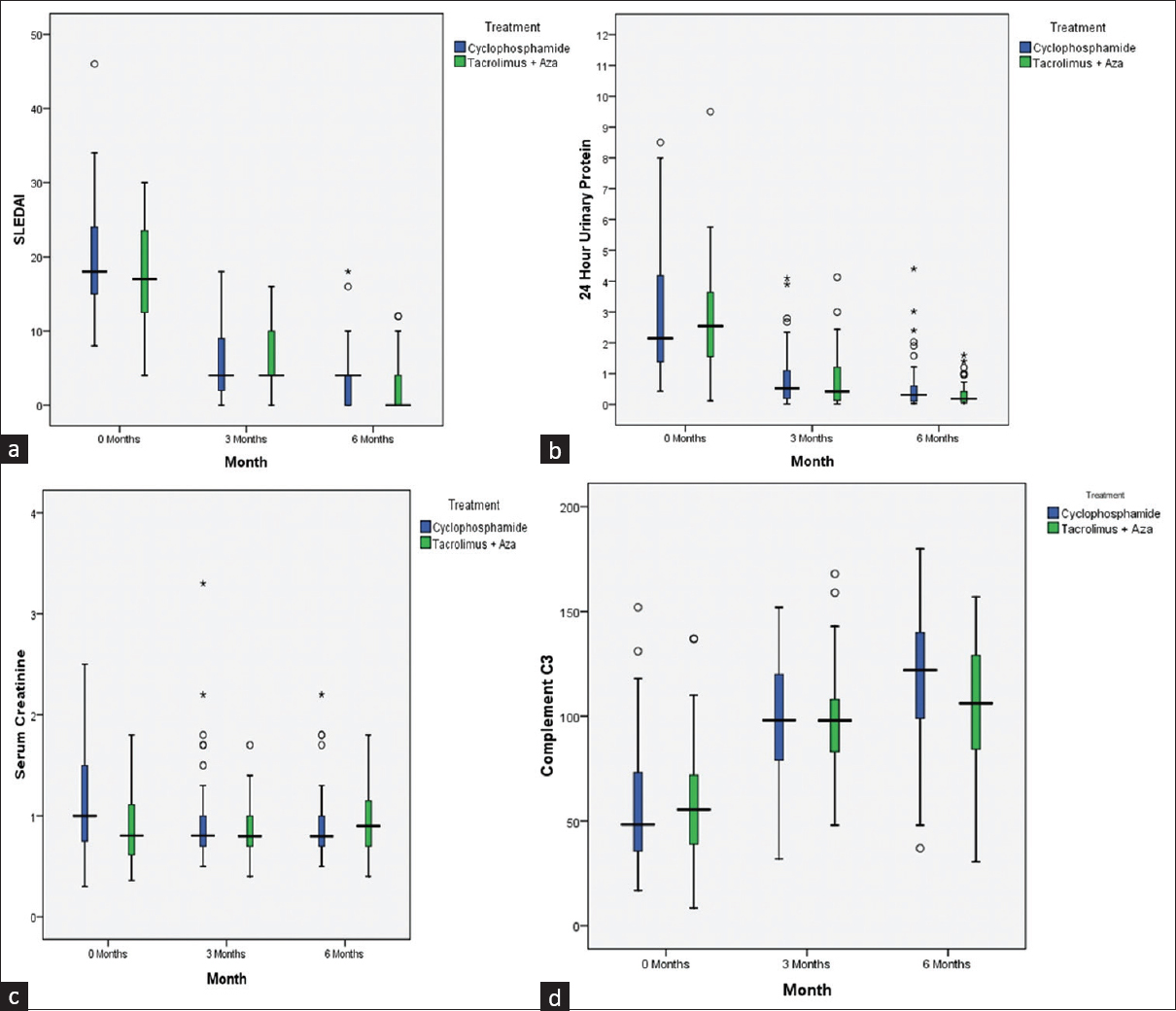
- (a–d) Box plots of SLEDAI (a), proteinuria (b), serum creatinine (c), and complement factor 3 (d) at baseline, 3 months, and 6 months in the MMTT group and IV CYC group. (a) There was significant improvement in SLEDAI score in both the arms from baseline to M3 and M6. The mean reduction of SLEDAI score in the two arms was similar at 3 and 6 months, respectively. (b) There was significant improvement in 24-h urinary protein in both the arms from baseline to month 3 and month 6. (c) Serum creatinine level was higher at baseline in the CYC arm, which became similar at 3 months in both the arms, but at 6 months, the mean creatinine level of the multitarget arm was higher than the CYC arm. (d) Serum C3 level recovery was significantly better in both the arms compared to baseline and at month 3 and month 6, and remained comparable in both the arms at the end of 6 months. CYC = cyclophosphamide, IV = intravenous, MMTT = modified multitarget therapy, SLEDAI = systemic lupus erythematosus disease activity index
Twenty-nine out of 44 (65.9%) in the MMTT arm and 30 out of 49 (61.2%) in the IV CYC arm achieved CR. PR occurred in nine more patients in the MMTT arm and 13 more patients in the IV CYC arm. Overall remission (CR + PR), that is, the primary outcome, was comparable in both the arms: MMTT (86.36%) and IV CYC (87.75%) [Table 3]. Median 24-h urine protein at the end of the study improved satisfactorily in both the arms, without any statistically significant difference between the two arms (0.19 vs. 0.31 g/day, P < 0.09). More patients in the MMTT arm achieved CR (65.9% vs. 61.2%). Serum creatinine at the end of 6 months was significantly better in the IV CYC group. Though statistically different, the creatinine value was practically within normal limit in both the arms (MMTT arm 0.96 mg/dl vs. IV CYC arm 0.92 mg/dl). Calcineurin inhibitors (CNI) may increase creatinine because of their hemodynamic effect and/or parenchymal injury. Three patients in the triple drug group showed increase of creatinine, which improved with dose adjustment. Nine patients (20.5%) in the triple drug group and only two (4.1%) patients in the IV CYC group showed increase in creatinine to more than 30%. Among these nine patients, seven patients achieved proteinuria remission and disappearance of active sediments with stabilization of creatinine following tacrolimus dose adjustment, while two patients could not achieve proteinuria remission, either CR or PR, within 6 months. In the IV CYC group, six patients did not achieve any remission at the end of 6 months; two of them showed increase in creatinine and all six had persistence of active sediments in urine.
| MMTT arm (n=44) | SOC arm (n=49) | P | |
|---|---|---|---|
| Overall response (CR+PR) | 38/44 (86.36%) | 43/49 (87.75%) | 1 |
| 24-h urine protein Median (interquartile range) (g/day) | 0.19 (0.10-0.42) | 0.31 (0.11-0.60) | 0.09 |
| Serum creatinine (mg/dl) | 0.96 (0.34) | 0.92 (0.35) | 0.64 |
| Proteinuria remission (CR) | 29/44 (65.90%) | 30/49 (61.22%) | 0.32 |
| Active sediment | 2/44 (4.5%) | 6/49 (12.2.%) | 0.27 |
| SLEDAI score | 2.75 (3.60) | 3.20 (3.80) | 0.56 |
| C3 level (mg/dl) | 104.33 (28.96) | 117.66 (30.08) | 0.03 |
| C4 level (mg/dl) | 22.96 (10.37) | 22.43 (9.76) | 0.80 |
CR=Complete remission, MMTT=Modified multitarget therapy, PR=Partial remission, SLEDAI=Systemic lupus erythematosus disease activity index
Secondary outcome
The results for the secondary outcomes are presented in Figure 2a and d.
The SLEDAI score, reflecting the disease activity, changed significantly in both the groups at the end of 6 months. Both the groups were comparable in terms of reduction (2.75 [3.60] vs. 3.20 [3.80]). C3 improvement, which indicates decline in disease activity, improved in both the arms. There was no flare in either group. Five patients in each group failed to achieve either CR or PR.
Major AEs were numerically less in the MMTT arm. Five major events were registered. Two patients had pneumonia with transient creatinine increase, which improved with antibiotics and tacrolimus dose adjustment. One patient was admitted with multiple abscesses in thigh and chest wall and improved with conservative management. One patient had transient cytopenia, which improved with decrease in the dose of azathioprine to 1.5 mg/kg body weight. The IV CYC (8/52 [15.38%]) arm had six patients with pneumonia requiring hospitalization. All of them improved, except one who succumbed. Two patients had developed PRES and responded to escalation of antihypertensive drugs. With regard to minor AEs, in the MMTT arm, there were 10 recorded events (20.83%). One each had vomiting, superficial fungal infection, urinary tract infection, and hyperglycemia and six patients had new-onset hypertension. Thirteen such minor events were seen in the IV CYC arm. One patient each had scabies, Herpes Zoster, and hyperglycemia, while four patients had upper respiratory tract infection, which improved with oral antibiotics. Also, four patients had reported vomiting and abnormal liver function tests LFT was recorded in two of them. AEs were not significantly different in both the arms. The IV CYC arm had more infections requiring hospital admission, including one death.
Discussion
To our knowledge, this study is the first single-center, randomized trial to report modified multitarget combination regimen (MMTT) comprising azathioprine, tacrolimus, and steroids for PLN. Based on the observations from MMF versus azathioprine use in lupus nephritis and transplant field,[1112] we propose several advantages for substitution of MMF by azathioprine, such as reduction of sepsis risk and continuation of the drug regimen in case of accidental pregnancies. As an Indian public sector hospital, we observed higher noncompliance with MMF compared to azathioprine. This may possibly be attributed to multiple dosing strategies and higher cost of MMF.
The goal of LN therapy is to attain successful reduction of proteinuria, a surrogate marker of good long-term outcome. Remission of proteinuria at the end of 6 months, be it complete or partial, improves 10-year renal as well as patient survival.[1314] In our study, we had shown CR in 65.9% of patients in the MMTT arm versus 61.2% in the IV CYC arm at the end of 6 months. Overall remission was also comparable in both the groups (MMTT arm 86.36% vs. 87.75% in the IV CYC arm), with comparable recovery of complement levels and reduction in SLEDAI score in both the arms at the end of 6 months. Most of the studies which looked at predictive factors for poor prognosis in LN had documented persistently low complement factors (C3 or C4)[14] as one important predictor. MMTT arm had been able to achieve this benefit of stabilizing complement factors at 3 months.
The first reported multitarget trial by Bao et al.[14] and the largest multicenter Hong Kong Group trial[9] both had shown high overall response rates, which were close to our observations. Bao et al. reported that out of 18 patients assigned to each arm, 16 (88.9%) patients assigned to multitarget therapy and nine (50.0%) patients assigned to IV CYC achieved either CR or PR. Similarly, the Hong Kong group published the overall (CR and PR) response at week 24 to be 83.5% in the multitarget treatment group and 63.0% in the IV CYC group and a shorter median time to response in the multitarget group (8.9 weeks) than in the IV CYC group (13 weeks). The IV CYC group in a Chinese study showed worse outcomes than what we had seen in our study. This could be due to the inclusion of class V lupus patients in both Chinese MMF-based multitarget studies. Pure class V lupus nephritis subgroup analysis in the Hong Kong study had shown much higher percentage point difference in proteinuria improvement in the multitarget arm than in the IV CYC group (33.1% vs. 7.8%; difference 25.3 percentage points), while pure class IV and a mix of IV + V LN had relatively less percentage point difference between the two arms. Earlier studies by Bao et al.[14] and Najafi et al.[15] documented relatively poor response with CYC treatment when class V lupus nephritis was compared with class IV. While designing our trial, we excluded patients with pure membranous lupus who are likely to have relatively higher proteinuria and nephrotic range presentation and may benefit from nonimmune direct podocyte cytoskeleton-stabilizing property and antiproteinuric effect of tacrolimus.[16]
The use of CNI has been extensively explored in LN in an attempt to improve overall remission rate. We targeted a lower C0 level to minimize acute tacrolimus-related nephrotoxicity, which was maintained at 5–8 ng/ml. Chen et al.[17] treated class IV and V LN patients with tacrolimus, also targeting a T0 of 5–10 ng/ml, and reported complete remission at 6 months in 52% of tacrolimus-treated patients and 38% of CYC-treated patients, with most failures observed due to persistent proteinuria. Later, another study from non-Asian background also confirmed the efficacy of tacrolimus[18] in LN. One meta-analysis exploring the evidence for tacrolimus use in PLN opined that overall, tacrolimus was more effective at inducing complete renal remission than IV CYC (P = 0.004), but there was no significant difference compared to MMF (P = 0.87).[19] This can be justified by podocyte protective property of tacrolimus.[20] A recent trial with voclosporin, another CNI, proves the class effect of CNI in improving outcome.[21] Combining CNI with other antimetabolites may reduce the dose of a single agent, while maintaining similar net immunosuppression, and may improve the efficacy, maximize safety, and translate to better long-term outcome.
Bargman et al.[22] had pointed out two big advantages of azathioprine in comparison to MMF; while retaining comparable efficacy and safety, azathioprine is 10–15 times cheaper and could be used in pregnant lupus patients, which was a need for this young cohort. In our study, two patients in the MMTT arm conceived and were excluded from the analysis. Poor socioeconomic status, increased chance of noncompliance, and risk of sepsis came out as predictive factors of poor outcome of lupus in the real-world scenario.[23] The Mycophenolate Steroids Sparing (MYSS) study[24] and another Indian study[25] in transplant population had shown azathioprine to be noninferior to MMF in terms of efficacy as well as safety. Similar observations were reported by a Cochrane database meta-analysis,[26] that is, comparable graft and patient survival at long-term follow-up with more tissue-invasive CMV infection in the MMF arm. No study compared MMF with azathioprine as part of induction regimen in LN. The maintenance regimen trials have shown variable results in terms of efficacy and safety, which indicates more trials are necessary. At present, in this trial, replacement of MMF with azathioprine did not compromise the efficacy or safety of the regimen. Long-term outcome difference, if any, may be evident in the maintenance therapy period.
We did not provide biopsy outcome at the end of 6 months because in one of our previously published studies, we have shown that only 25% of biopsies did have class change while protocol biopsy was done at 6 months and the rest did not show much change, even if clinical and biochemical remission are achieved.[27] So, we planned to have biopsy at 1 year before withdrawing tacrolimus.
Contreras et al.,[28] in their long-term maintenance, noted significantly higher hospitalization rate with more severe infections, more amenorrhea, and more gastrointestinal (GI) side effects with IV CYC when compared to MMF or azathioprine arm. The Hong Kong group showed more AEs and dropouts in the multitarget group than in the IV CYC arm (serious AEs: multitarget 7.2% [13 of 181] vs. IV CYC 2.8% [5 of 181]; dropouts due to adverse effects: multitarget vs. CYC 5.5% vs. 1.7%, P = 0.086).
Two patients in the MMTT arm were lost to follow-up, while we reported one death and three patients lost to follow-up in the standard of care IV CYC arm. Both groups also had no significant differences in terms of frequency of hospitalization, amenorrhea, infection, and GI side effects. New-onset hypertension was more in the MMTT group (six vs. two), which may be due to the combination of tacrolimus and steroids. Similar observation had been recorded by another multitarget trial.[9]
Limitations of our study are that it is a single-center study and severe lupus was excluded. We looked at the improvement of surrogate markers like proteinuria, estimated glomerular filtration rate (eGFR), and the SLEDAI score, but not repeat biopsy, which is planned later in our study protocol. This study reports the short-term outcome, and the impact of induction therapy usually influences the maintenance phase as well.
Conclusion
Lupus nephritis is a heterogeneous disease and the outcome depends on demographic, racial, histopathologic, serological, and socioeconomic factors. Appropriate therapeutic decision often needs assessment of these multiple factors to ensure better long-term outcome. The MMTT arm, as induction therapy, appeared to have comparable effectiveness and safety with the IV CYC arm, the standard of care induction regimen, in PLN, with a reasonably good short-term improvement in CR and PR.
Financial support and sponsorship
Department of Nephrology.
Conflicts of interest
There are no conflicts of interest.
References
- Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: A systematic review and bayesian meta-analysis. Arthritis Rheumatol. 2016;68:1432-41.
- [Google Scholar]
- Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from1995 to 2006. Arthritis Rheum. 2011;63:1681-8.
- [Google Scholar]
- The natural history of the renal manifestations of systemic lupus erythematosus. 1964. J Am Soc Nephrol. 1997;8:1189-98.
- [Google Scholar]
- Corticotropin and cortisone in acute disseminated lupus erythematosus: Results of long-term use. JAMA. 1952;149:1002-8.
- [Google Scholar]
- Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614-9.
- [Google Scholar]
- Aspreva lupus management study group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20:1103-12.
- [Google Scholar]
- Clinical features, epidemiology, and short-term outcomes of proliferative lupus nephritis in Eastern India. Indian J Nephrol. 2013;23:5-11.
- [Google Scholar]
- Treatment of severe lupus nephritis: The new horizon. Nat Rev Nephrol. 2015;11:46-61.
- [Google Scholar]
- Multitarget therapy for induction treatment of lupus nephritis: A randomized trial. Ann Intern Med. 2015;162:18-26.
- [Google Scholar]
- Triple drug regimen as induction treatment of lupus nephritis: A pilot randomized controlled trial. Int J Nephrol Ther. 2018;4:7-11.
- [Google Scholar]
- Maintenance therapy for lupus nephritis with mycophenolate mofetil or azathioprine. A meta-analysis. Clin Nephrol. 2019;91:172-9.
- [Google Scholar]
- Mycophenolate versus azathioprine for kidney transplantation: A 15-year follow-up of a randomized trial. Transplantation. 2012;94:152-8.
- [Google Scholar]
- Relapses of lupus nephritis: Incidence, risk factors, serology and impact on outcome. Lupus. 2003;12:692-6.
- [Google Scholar]
- Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol. 2008;19:2001-10.
- [Google Scholar]
- Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney Int. 2001;59:2156-63.
- [Google Scholar]
- Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS One. 2015;10:e0132724.
- [Google Scholar]
- Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: A multicenter randomized clinical trial. Am J KidneyDis. 2011;57:235-44.
- [Google Scholar]
- Tacrolimus in non-Asian patients with SLE: A real-life experience from three European centres. Lupus Sci Med. 2018;5:e000274.
- [Google Scholar]
- Multitarget therapy versus intravenous cyclophosphamide in the induction treatment of lupus nephritis: A metaanalysis of randomized controlled trials. Turk J Med Sci. 2018;48:901-10.
- [Google Scholar]
- Calcineurin inhibitors in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2017;31:429-38.
- [Google Scholar]
- A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;95:219-31.
- [Google Scholar]
- How did cyclophosphamide become the drug of choice for lupus nephritis? Nephrol Dial Transpl. 2009;24:381-4.
- [Google Scholar]
- Prognosis in proliferative lupus nephritis: The role of socio-economic status and race/ethnicity. Nephrol Dial Transplant. 2003;18:2039-46.
- [Google Scholar]
- Mycophenolate mofetil versus azathioprine therapy is associated with a significant protection against long-term renal allograft function deterioration. Transplantation. 2003;75:1341-60.
- [Google Scholar]
- Long-term graft outcome with mycophenolate mofetil and azathioprine: A paired kidney analysis. Transplantation. 2006;82:1634-9.
- [Google Scholar]
- Comparison of azathioprine with mycophenolate mofetil in a living donor kidney transplant programme. Indian J Nephrol. 2011;21:258-63.
- [Google Scholar]
- Protocol renal biopsy in patients with lupus nephritis: A single center experience. Saudi J Kidney Dis Transpl. 2014;25:801-7.
- [Google Scholar]
- Maintenance therapies for proliferative lupus nephritis: Mycophenolate mofetil, azathioprine and intravenous cyclophosphamide. Lupus. 2005;14((Suppl 1)):s33-8.
- [Google Scholar]







