Translate this page into:
Clinical Trial Comparing the Efficacy and Safety of Regional Citrate Anticoagulation Versus Heparin in CRRT
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Heparin continues to be the most common modality of anticoagulation in CRRT. The increased risk of hemorrhagic complications associated with its use led to the emergence of regional citrate anticoagulation (RCA) as an alternative. However, the perceived complexities associated with its use and the risk of metabolic derangements have prevented it from being adopted on a larger scale. Thus, we conducted a prospective study to compare the efficacy and safety of RCA versus heparin.
Methods:
Adult patients admitted to our ICU (November 2018–November 2019) with renal insufficiency and requiring CRRT were included in the study. It was an open-label study with 25 patients each being allotted to the heparin and citrate groups. Our primary outcome was the filter life span. Secondary outcomes included metabolic derangements, bleeding episodes, and patient survival. The starting dose of citrate was 2.0 mmol/L.
Results:
The mean filter life span was 32.84 h in the citrate group and 30.40 h in the heparin group (p-value = 0.47). In a significant proportion of the cases, CRRT was terminated for non-filter clotting-related reasons (64% in citrate vs. 32% in heparin). Kaplan–Meir analysis was done to overcome this confounder; the filter lifespan was estimated to be 46.94 h in citrate and 40.05 h for the heparin group (p-value = 0.29). No significant metabolic derangements or bleeding episodes were noted in either group. Overall patient survival was higher in the citrate group at 52% versus 32% (p-value = 0.15) in the heparin group.
Conclusion:
No significant difference in filter lifespan or risk of metabolic derangements was noted. A trend toward higher patient survival rates in the citrate group was noted, which warrants further evaluation in future trials.
Keywords
Citrate anticoagulation
CRRT
RCA
Introduction
Continuous renal replacement therapy (CRRT) is used for the management of kidney dysfunction in critically ill patients with hemodynamic instability. Heparin is the most commonly used modality for circuit anticoagulation but carries with it a significant risk of hemorrhagic complications. The search for alternative methods of anticoagulation led to the introduction of regional citrate anticoagulation (RCA) almost two decades back.
Citrate, the anionic salt of citric acid, anticoagulates the extracorporeal circuit by chelating ionized calcium (a key cofactor for many steps in the clotting cascade).[123] Citrate also has multiple other potential effects, including reduced activation of white blood cells and platelets and protective effects against endothelial cell inflammation and dysfunction. Finally, citrate also represents a source of energy (0.59 kcal/mmol).[1]
Citrate is infused in the initial portion of the circuit at rates proportional to blood flow and is then titrated to maintain low ionized calcium levels in the extracorporeal circuit sufficient enough to achieve full blood anticoagulation (0.3–0.4 mmol/L); this target is generally attained with a citrate level in the extracorporeal circuit of approximately 3 mmol/L2.[23] Because both citrate anion and calcium-citrate complexes are lost in the effluent fluid, calcium infusion is needed to replace ongoing calcium losses and maintain systemic ionized calcium levels in the normal range.
Citrate accumulation continues to be the most common complication of RCA, with a reported incidence of 0%–12% of patients depending on the RCA protocol used.[4567891011121314] In clinical conditions such as severe liver failure or septic/cardiogenic shock, reduced citrate metabolism may prevent bicarbonate generation from citrate, leading to metabolic acidosis as well as a considerable fall in ionized serum calcium levels to chelation of calcium. As a consequence, progressively higher calcium infusion rates may be required to maintain the ionized calcium concentration within physiologic limits, and there may be a disproportionate rise in both the total systemic calcium concentration and the total-to-ionized calcium ratio (the calcium ratio).[23814] In clinical practice, this calcium ratio is accepted as an indirect index of citrate accumulation during RCA.[11] A ratio of over 2.5 is considered the critical threshold for increased risk of metabolic complications caused by impaired citrate metabolism. The main risk of citrate accumulation is hypocalcemia, which can lead to hypotension and arrhythmias. In these clinical settings, methods to prevent citrate accumulation should be followed to reduce the citrate load by decreasing citrate administration (lower blood flow rates and higher ionized calcium targets) and/or increasing citrate clearance (higher convective and/or diffusive dialysis dose).
The risk of other electrolyte and acid-base disorders, including hypernatremia, hypomagnesemia, and metabolic alkalosis, also exist but are quite uncommon when strict adherence to the RCA protocol is ensured.[15]
Although several studies have demonstrated the effectiveness of regional citrate anticoagulation, data from India regarding the same has been rather lacking. The perceived complexities associated with the citrate regimen and the risk of metabolic derangements have impeded universal adoption of this potentially superior modality. Our study was thus aimed at comparing the efficacy of unfractionated heparin with that of RCA in CRRT. To our knowledge, this is the first comparative study of such kind from India.
Materials and Methods
Study design and patient selection
This was a single center, prospective, open-label, comparative study. Patients admitted to our ICU between November 2018 and November 2019 with AKI (regardless of the cause) and requiring CRRT were included in the study. Patients under the age of 18 years, those with a systolic BP of <80 mm Hg, and those in whom CRRT was run for less than 6 h were excluded from the study.
Intervention
CVVHDF was done with Prismaflex Machine and M100 set (including an AN69 dialyzer). Blood flow, dialysate flow, and fluid removal rates were adjusted on a per-patient basis. Filtration fraction of <20% and effluent dose of ~25 mL/kg/h were targeted.
For citrate anticoagulation, Regiocit from Baxter (Na 140 mmol/L, Cl 86 mmol/L, Citrate 18 mmol/L) was used as a replacement fluid, and it also served as the source of citrate. Biphozyl from Baxter (Na 140 mmol/L, K 4 mmol/L, Cl 122 mmol/L, HCO3 25 mmol/L, HPO4 1 mmol/L, Mg 1 mmol/L) was used as dialysate. Owing to the dearth of Indian data regarding the RCA protocol, we erred on the conservative side and started at a lower citrate dosage of 2.0–2.5 mmol/L (in contrast to earlier studies with citrate doses of 2.5–4.5 mmol/L) and then titrated it based on post-filter ionized calcium levels (target: 1.2–2 mg/dL) measured at 6-h intervals. Prismaflex software comes with an integrated citrate infusion system that indicates the cumulative citrate dosage delivered to the patient (thus minimizing the risk of citrate toxicity and was targeted to be kept within 7–10 mmol/h). Calcium was replenished through a separate infusion pump (undiluted calcium gluconate in a 50-mL infusion syringe). It was started at 12 mL/h (2.2 mmol/h) and then titrated as per systemic ionized calcium levels (target: 3.6–4.8 mg/dL) measured at 6-h intervals. As part of the citrate protocol, post-filter and systemic-ionized calcium was measured at 6-h intervals (from ABG). Serum total calcium, magnesium, phosphate, urea, and creatinine were measured daily.
For heparin anticoagulation, Hemosol from Baxter was used as the dialysate and replacement fluid. Heparin was infused after the blood pump and before the hemofilter. A bolus of 2000–5000 U was injected into the circuit at the commencement of CVVHDF (exact dose being based on patient size and preexisting aPTT). An infusion of heparin was commenced simultaneously at an initial rate of 200–500 units/h and adjusted to maintain APTT at 60–80 s in the patient (aPTT to be determined every 4 h). Serum potassium was monitored on a 4-h basis.
Outcomes
The primary outcome was filter lifespan [measured from the time of commencement of CRRT to the time of either elective discontinuation, circuit clotting, or persistently high transmembrane pressure (>300 mm Hg) prohibiting the continuation of the therapy]. There was no predefined limit to the time that a hemofilter could be used.
Secondary outcomes included incidence of bleeding episodes (defined as the occurrence of any systemic or local bleeding during CRRT), metabolic derangements (including metabolic acidosis, metabolic alkalosis, hypernatremia, hyponatremia, hypocalcemia), and patient survival.
Data collection and analysis
Data were initially entered and collated in MS-EXCEL. Statistical analysis was performed using SPSS (version 26) software package. Categorical variables are presented as numbers and percentages and continuous variables as mean ± standard deviation. Continuous outcomes that were normally distributed were compared using a t test. Proportions were compared using a Chi-square test. A plot of the Kaplan–Meier estimate for the survival function of each subject’s filter was performed, and the circuit lifespan in the two groups was compared using a log-rank test.
Ethical clearance
Prior to its initiation, a brief of the study (including the methodology) was presented to our institutional ethics committee and due clearance was obtained. Informed patient consent was obtained. The study was conducted in strict adherence to the ethical principles in the declaration of Helsinki.
Results
Baseline characteristics
Baseline characteristics [Table 1] between the two groups were comparable with no statistically significant differences. APACHE 2 grading was done at the time of admission to assess baseline disease severity. Septic shock accounted for the majority of the cases, with 76% and 80% in the heparin and citrate groups, respectively. Type 2 DM was the most common comorbid condition in both groups, followed by ischemic heart disease and hypertension.
| Heparin | Citrate | P | |
|---|---|---|---|
| Age | 52.92±17.16 | 47.84±16.09 | 0.29 |
| Sex | |||
| Male | 17 | 19 | 0.53 |
| Female | 08 | 06 | |
| Diagnosis | |||
| Septic Shock | 19 | 20 | |
| Cardiogenic Shock | 02 | 01 | |
| Post-Operative | 01 | 03 | |
| Others | 02 | 01 | |
| Apache ii grading | 0.79 | ||
| Score | 18.68±5.9 | 19.08±4.7 | |
| Mortality risk (%) | 32 | 28 | |
| Mechanical ventilation | |||
| Yes | 19 | 18 | |
| No | 06 | 07 | |
| Creatinine (at the start in mg/dL) | 5.46±2.54 | 5.69±3.01 | 0.77 |
| Comorbidities | |||
| Type 2 DM | 12 | 10 | |
| Hypertension | 06 | 06 | |
| Ischemic heart disease | 06 | 07 | |
| Chronic kidney disease | 03 | 03 | |
| Cerebrovascular disease | 01 | 02 | |
| Hypothyroidism | 01 | 02 | |
| Malignancy | 02 | 01 |
Filter lifespan
The citrate group with a filter lifespan (regardless of the reasons for stoppage of CRRT) of 32.84 h fared slightly better than the heparin group at 30.40 h, but this difference did not achieve a statistical difference (p-value = 0.474). Reasons for discontinuation of CRRT [Table 2] among the two groups were varied, and there was a statistically significant difference between the two groups (p-value = 0.047). Improvement in patient’s metabolic parameters or hemodynamic status (64%) was the most common reason for stoppage of CRRT in the citrate group, while filter clotting (48%) was the most common reason for stoppage in the heparin group. In addition, more number of CRRT sessions were terminated owing to the death of a patient in the heparin group (20%) than in the citrate group (4%). Consequently, Kaplan–Meir survival analysis [Figure 1 and Table 3] was done to assess the cumulative filter survival after censoring for discontinuation of CRRT due to reasons other than clotting of the filter. The mean estimated filter lifespan (after censoring for non-clotting-related discontinuations) from the above analysis was 46.94 h for the citrate group and 40.05 h for the heparin group (p-value = 0.29).
| Filter Clot | Improved | Expired | |
|---|---|---|---|
| Citrate (n=25) | 08 (32%) | 16 (64%) | 01 (4%) |
| Heparin (n=25) | 12 (48%) | 08 (32%) | 05 (20%) |
Chi-Square test: χ2=6.133; P=0.04, Likelihood ratio: χ2=6.435; P=0.04
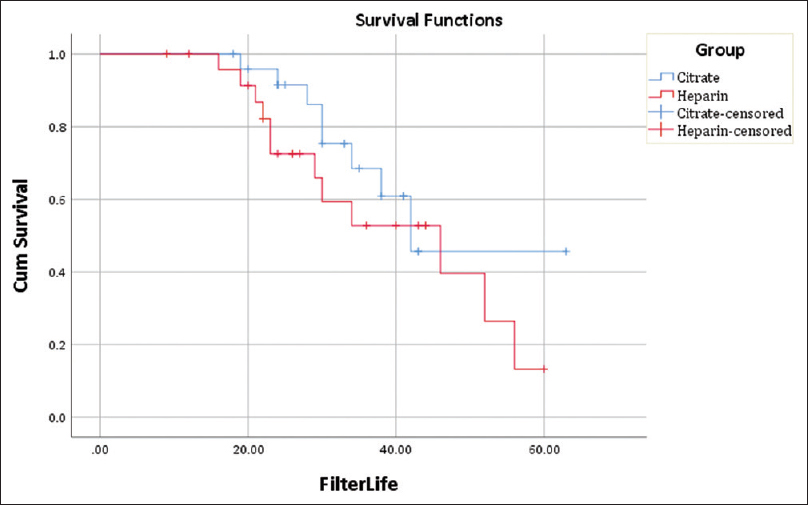
- Kaplan–Meir analysis of expected filter lifespan
| Tests of Significance | Chi-square | Significance |
|---|---|---|
| Log Rank (Mantel-Cox) | 1.087 | 0.297 |
| Breslow (Generalized Wilcoxon) | 1.875 | 0.171 |
| Tarone-Ware | 1.467 | 0.226 |
Metabolic derangements and bleeding
No significant differences were noted in pH, lactate, bicarbonate, and magnesium levels [Table 4]. Ionized calcium levels were lower in the citrate group and potassium levels were lower in the heparin group. Neither parameters were low enough to cause any significant clinical manifestations. No significant bleeding episodes were noted in either group.
| pH | Lactate | HCO3 | K | Ca | Mg | |
|---|---|---|---|---|---|---|
| Citrate | ||||||
| Mean | 7.33 | 2.89 | 17.32 | 4.02 | 3.64 | 1.99 |
| Std. Dev | 0.07 | 3.57 | 2.91 | 0.68 | 0.32 | 0.17 |
| Heparin | ||||||
| Mean | 7.32 | 2.93 | 16.80 | 3.29 | 3.88 | 2.04 |
| Std. Dev | 0.07 | 1.94 | 2.99 | 0.31 | 0.40 | 0.15 |
| Total | ||||||
| Mean | 7.32 | 2.91 | 17.06 | 3.65 | 3.76 | 2.02 |
| Std. Dev | 0.07 | 2.84 | 2.93 | 0.64 | 0.38 | 0.16 |
| Significance (ANOVA) | 0.494 | 0.953 | 0.536 | 0.000012 | 0.024 | 0.372 |
Patient survival
Overall patient survival was higher in the citrate at 52% versus 32% (p-value = 0.15) in the heparin group [Figure 2].
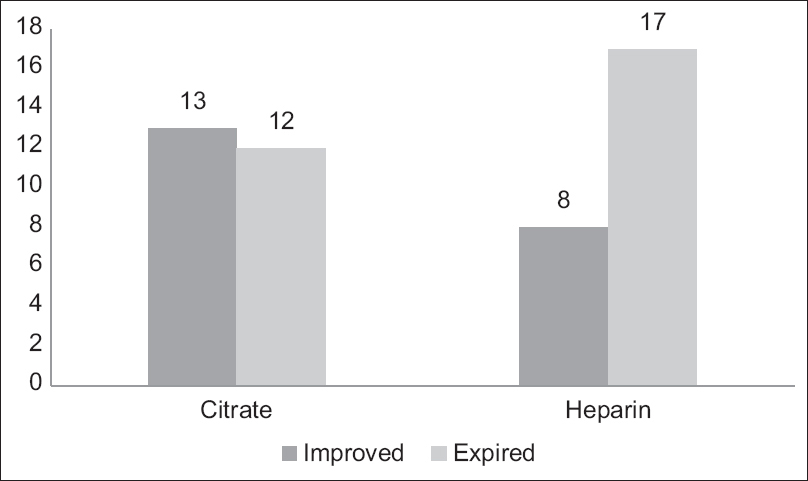
- Comparison of patient survival
Discussion
The introduction of regional citrate anticoagulation for dialysis dates back over a couple of decades. Several studies since then have gone on to demonstrate the efficacy and safety of citrate relative to heparin. These led to the KDIGO recommending RCA as the anticoagulant modality of choice for CRRT in the absence of any contraindications. Despite these advances, heparin continues to be the predominant choice in most Indian hospitals, largely due to the perceived risk of metabolic complications and additional complexities with the use of citrate protocol.
With respect to filter efficacy, our study reported findings similar to most other studies. The mean filter life span in the citrate group at 32.84 h was slightly higher than in the heparin group at 30.40 h (difference statistically not significant). In a significant proportion of cases in both groups, however, CRRT was terminated for reasons other than filter clotting, either voluntarily (48%) or because the patient expired (12%). The inclusion of these cases in the calculation of mean filter lifespan resulted in a relatively lower filter life span compared to other studies.
Filter clotting was the reason for discontinuation of CRRT in only 32% of cases in the citrate group and 48% in the heparin group. More CRRT sessions were terminated voluntarily (either because the patient’s renal parameters or hemodynamic status improved) in the citrate group (64%) than in the heparin group (32%). In contrast, CRRT discontinuations secondary to the death of the patient were higher in the heparin group (20%) than in the citrate group (4%). The difference in these outcomes between the two groups was statistically significant with a P value of 0.047.
To overcome the abovementioned confounding effects and to derive an estimate of the expected filter life after censoring for non-clotting-related discontinuations, we did a Kaplan–Meir survival analysis. The mean filter lifespan from the analysis was estimated to be 46.94 h for citrate and 40.05 h for heparin (p-value = 0.29).
No major bleeding episodes were noted in either group in our study, which is in contrast to other trials, which reported higher bleeding risk in the heparin group. One plausible reason (and a limiting factor in our study) may be that quite a few patients in whom we perceived an increased risk of bleeding were intentionally assigned to the citrate group to avoid any untoward bleeding episodes. This may have led to a significant underestimation of the bleeding risk in the heparin group.
With regards to metabolic parameters in our study, hypokalemia was more common in the heparin group (3.3 mEq/L vs. 4.0 mEq/L), while there was a tendency toward hypocalcemia in the citrate group (3.6 mg/dL vs. 3.8 mg/dL). However, neither derangements were severe enough to cause any clinical manifestations. This was in line with previous studies which reported a greater incidence of asymptomatic hypocalcemia in the citrate group. We did not observe any increased incidence of metabolic alkalosis or acidosis in the citrate group, as was noted in some other studies.
Overall survival was higher in the citrate group at 52% than in the heparin group at 32%. Some of the earlier studies also demonstrated similar trends but failed to achieve statistical significance (as was the case in our study as well). In addition, as discussed earlier, the citrate group witnessed a greater proportion of CRRT sessions being terminated voluntarily secondary to improvement in the patient’s condition. These outcomes taken together highlight the need to further evaluate the effect of citrate on overall patient survival in bigger studies.
One of the major hindrances to the widespread adoption of citrate protocol has been the lack of clear guidelines regarding the starting dosage of citrate and titration protocols. Starting citrate concentration in previous studies ranged from 2.5 to 4.5 mmol/L (with most veering toward the higher side). It was then titrated to achieve post-filter ionized calcium of 1–1.5 mg/dL. As most of the potential complications in RCA were related to citrate itself and as experience with Indian patients was limited, we started at a lower citrate dose of 2 mmol/L and targeted a wider post-filter calcium range of 1.2–2 mg/dL. Interestingly, this citrate dosage proved sufficient enough to maintain adequate circuit anticoagulation in most cases. The lower dosage of citrate probably accounted for the lower incidence of metabolic complications in our study.
Study Limitations
Ours was a non-randomized and open-label study, which may have resulted in the introduction of an allocation bias (especially in the comparison of bleeding risk). Being a single-center study, our sample size was relatively small and was not adequately powered for evaluation of some of the secondary variables under study. Because many of the CRRT sessions were voluntarily terminated at around 24–36 h, calculation of actual filter lifespan was hampered and consequently, filter lifespans in our study were lower than in other such trials.
Conclusion
To summarize, our study reiterates that citrate at the very least is on par with heparin in terms of filter lifespan, with no added risk of metabolic complications. In addition, a lower dose of citrate (2.0–2.5 mm/L) may be enough, especially in the Indian context and helps to bolster the safety profile of the RCA protocol. The advantage in patient survival in the citrate group noted in our study was another encouraging aspect that needs further evaluation in future studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bench-to-bedside review:Citrate for continuous renal replacement therapy, from science to practice. Crit Care. 2012;16:249.
- [Google Scholar]
- Advances in continuous renal replacement therapy:Citrate anticoagulation update. Blood Purif. 2012;34:88-93.
- [Google Scholar]
- Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2:439-47.
- [Google Scholar]
- Regional citrate anticoagulation in cardiac surgery patients at high risk of bleeding:A continuous veno-venous hemofiltration protocol with a low concentration citrate solution. Crit Care. 2012;16:R111.
- [Google Scholar]
- Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int. 2005;67:2361-7.
- [Google Scholar]
- Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37:545-52.
- [Google Scholar]
- Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration:A prospective randomized multicentre trial. Nephrol Dial Transplant. 2011;26:232-9.
- [Google Scholar]
- Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med. 2001;29:748-52.
- [Google Scholar]
- A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Crit Care Med. 2009;37:2018-24.
- [Google Scholar]
- Normal citratemia and metabolic tolerance of citrate anticoagulation for hemodiafiltration in severe septic shock burn patients. Intensive Care Med. 2010;36:1735-43.
- [Google Scholar]
- Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit Care. 2012;16:R97.
- [Google Scholar]
- Regional citrate anticoagulation for high volume continuous venovenous hemodialysis in surgical patients with high bleeding risk. Ther Apher Dial. 2013;17:202-12.
- [Google Scholar]
- Continuous venovenous haemofiltration with citrate-buffered replacement solution is safe and efficacious in patients with a bleeding tendency:A prospective observational study. BMC Nephrol. 2013;14:89.
- [Google Scholar]
- Efficacy and safety of a citrate-based protocol for sustained low efficiency dialysis in AKI using standard dialysis equipment. Clin J Am Soc Nephrol. 2013;8:1670-8.
- [Google Scholar]
- Regional citrate anticoagulation for RRTs in critically Ill patients with AKI. Clin J Am Soc Nephrol. 2014;9:2173-88.
- [Google Scholar]







