Translate this page into:
AA Amyloidosis Presenting as Crescentic Glomerulonephritis
Address for correspondence: Dr. Praveen K. Etta, Consultant Nephrologist, Department of Nephrology and Renal Transplantation, Virinchi Hospitals and Max Superspeciality Medical Centre, Hyderabad, Telangana, India. E-mail: drpraveen85@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Renal amyloidosis is characterized by a non-proliferative pattern of injury with deposition of amyloid fibrils leading to massive proteinuria, and renal failure is usually mild and slowly progressive. Crescentic glomerulonephritis (CrGN) superimposed on amyloidosis has been reported very rarely. The majority of these cases belong to AA-type amyloidosis with proposed nonimmune pathogenesis due to the absence of immune deposits. The coexistence of immune complex-mediated diffuse proliferative glomerulonephritis (ICDPGN) with mesangial and endocapillary hypercellularity in these cases is extremely uncommon and low serum complement levels have not been reported in the past. Herein, we report a patient with rapidly progressive renal failure (RPRF) who presented with massive proteinuria, active urine sediments, and low serum complement level; renal biopsy was suggestive of immune complex mediated CrGN superimposed on AA amyloidosis.
A 62-year-old female presented with 3-weeks history of pedal edema and oliguria. On examination, she had pallor, pitting pedal edema, and high blood pressure (150/90 mmHg). The laboratory findings revealed hemoglobin of 9.2 g/dL, serum creatinine of 6.6 mg/dL (progressed from 2.3 mg/dL over 1 week), serum protein of 7.0 g/dL, and albumin of 3.2 g/dL. Her urine examination showed 3+ albumin, plenty erythrocytes/HPF, and 5–8 pus cells/HPF. Spot urine protein creatinine ratio was high (9.5 g/g). Serology for hepatitis B and C, human immunodeficiency virus (HIV), anti-nuclear antibody (ANA), rheumatoid arthritis (RA) factor, and anti-neutrophil cytoplasmic antibody (ANCA) was negative. Serum complement C3 level was significantly low (30 mg/dL; normal range 90–180 mg/dL); complement C4 and anti-streptolysin O (ASO) titer were normal. Renal biopsy findings on light microscopy revealed four out of eight glomeruli that were sclerosed. Three of the other glomeruli showed circumferential crescents which were cellular in one and fibrocellular in two. The underlying tuft was cellular with mesangial and endocapillary hypercellularity. Prominent eosinophilic, periodic acid–Schiff (PAS) – negative, silver – negative deposits were seen in the mesangium and walls of the blood vessels. The deposits were congophilic with apple-green birefringence on a polarized microscope confirming it as amyloid [Figure 1]. Mild interstitial fibrosis was noted. Immunofluorescence study showed significant peripheral and mesangial, granular deposits of IgG and C3c [Figure 2]. The deposits do not show light chain restriction. Hence, immunohistochemistry for AA amyloid was done and it was positive. Renal biopsy showed features of combined immune complex CrGN, and AA amyloidosis. After the biopsy report, she was treated with IV pulse steroids followed by oral steroids. Cyclophosphamide was not given due to a lack of consent. Further evaluation for underlying causes of AA amyloidosis was negative and testing for other organs' involvement including echocardiogram was negative. At last follow-up after 1 month, her creatinine was 5.0 mg/dL and she was dialysis independent.

- Top left and top right show two glomeruli with crescents. The tuft is hypercellular in the lower glomerulus and shows pale periodic acid–Schiff (PAS) negative, the silver negative matrix in the top glomerulus (PAS and PASM, ×40). Pale homogeneous deposits with the tinctorial characteristics of amyloid are also seen in the vessel wall at the bottom of the picture. The bottom left shows the apple-green birefringence of amyloid in the vessel wall and the glomerulus on the Congo red stain. The bottom right shows strong positivity of the amyloid for AA protein on immunohistochemistry
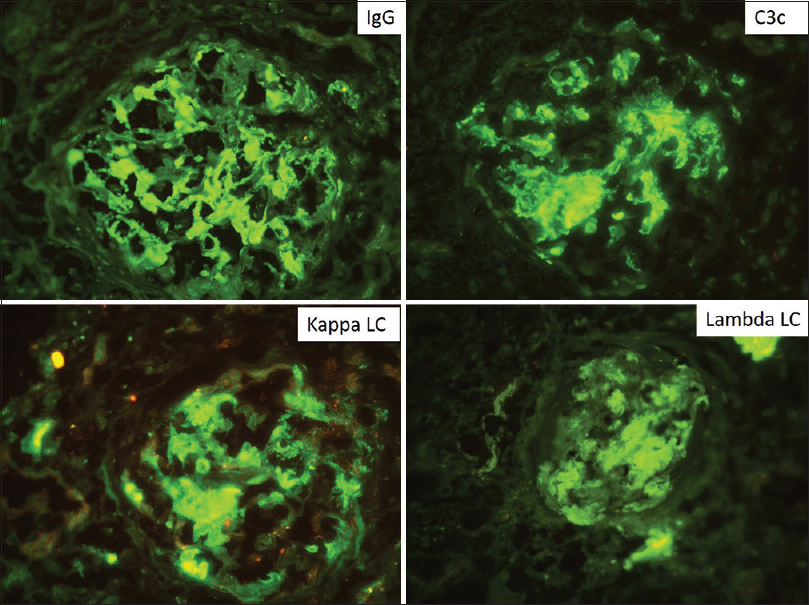
- Composite picture of direct immunofluorescence showing strong positivity in a peripheral and mesangial pattern for IgG, C3c, κ and λ light chains in equal intensity with no evidence of light chain restriction
Clinically, evident renal involvement mainly occurs in AL and AA amyloidosis. Laser microdissection followed by mass spectrometry-based proteomic analysis is the gold standard to identify the precursor protein in amyloidosis. In 1980, the first two cases of renal amyloidosis with CrGN were reported.[1] Since then around 20 cases of CrGN associated with amyloidosis were reported. The majority of these reported cases belong to AA amyloidosis, most often in the setting of RA.[2] The primary etiologies of AA amyloidosis include chronic infections and inflammatory conditions [Table 1]. In the largest case series till date, crescents were identified in up to 13% of renal amyloidosis (14 of 105) cases, often with >50% crescents (6 of 14 reported cases). AA type amyloid was detected in 12/14 cases (85%), RA was the most frequent underlying cause for it and a female predominance was noted (male:female, 3:11).[3] Recently two cases of cancer-associated AA amyloidosis presenting as CrGN have been reported.[4] The pathogenesis of crescent formation in amyloidosis is uncertain. The amyloid deposits are usually colocalized in areas of the glomerular basement membrane (GBM) rupture and crescents. Amyloid infiltration may result in mesangial cell dysfunction, increased GBM fragility, and damage with consequent leakage of fibrin into Bowman's space.[23] Most of the previously reported cases of amyloid complicated with CrGN have not shown significant mesangial or endocapillary hypercellularity with immune complexes deposition. Low complement levels have also not been described in these cases. Previous reports suggested a nonimmunological mechanism of crescentic proliferation due to a lack of detection of immune deposits and negative ANCA status.[5] It is unknown in our case whether the presence of multiple histological findings such as immune complex mediated CrGN, immune complexes deposits, and amyloidosis indicate coexistent multiple renal diseases or a single disease with a spectrum of findings. The treatment of AA amyloidosis is primarily directed at the underlying chronic inflammatory condition with the goal of decreasing the production of serum AA protein. Treatment of CrGN associated with amyloidosis is not clear. The benefit of immunosuppression with a combination of steroids and cytotoxic drugs is not well-documented, though it may benefit a few cases.[6] With no underlying secondary cause identified, our patient was treated with steroids, other supportive measures, and dialysis with only partial renal recovery, to reach dialysis independent state.
| Categories | Causes |
|---|---|
| Chronic infections | Tuberculosis, leprosy, bronchiectasis, chronic osteomyelitis, chronic pyelonephritis, Whipple's disease, subacute bacterial endocarditis, chronic skin or decubitus ulcers |
| Chronic inflammatory arthritis | Rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthropathy, reactive arthritis, adult Still's disease, SLE |
| Vasculitis | Polyarteritis nodosa, Takayasu arteritis, Behcet's syndrome, giant cell arteritis and polymyalgia rheumatica |
| Inflammatory bowel diseases | Crohn's disease, ulcerative colitis |
| Periodic fever | Familial Mediterranean fever, cryopyrin-associated periodic syndrome, TNF receptor-associated periodic syndrome |
| Neoplasia | Lymphoma, leukaemia, renal cell carcinoma, adenocarcinoma of lung, gut and urogenital tract, skin cancers |
| Others | Cystic fibrosis, hidradenitis suppurativa, IgG4-related disease, intravenous drug abuse |
SLE: Systemic lupus erythematosus, TNF: Tumour necrosis factor
To conclude, CrGN may be suspected in a case of renal amyloidosis (especially in AA type) with rapid worsening of renal function and it may coexist with features of ICDPGN affecting its clinical course, prognosis, and management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Rapidly progressive glomerulonephritis and possible amyloidosis. Arch Path Lab Med. 1980;104:603-9.
- [Google Scholar]
- Clinical and histological characteristics of renal AA amyloidosis: A retrospective study of 68 cases with a special interest to amyloid-associated inflammatory response. Hum Pathol. 2007;38:1798-809.
- [Google Scholar]
- Glomerular crescents in renal amyloidosis: An epiphenomenon or distinct pathology? Pathol Int. 2001;51:179-86.
- [Google Scholar]
- Cancer-associated AA amyloidosis presenting as crescentic glomerulonephritis. Kidney Int Rep. 2019;4:882-7.
- [Google Scholar]
- Renal failure in a patient with two renal diseases: Renal amyloidosis and rapidly progressive glomerulonephritis. Nephrol Dial Transplant. 1997;12:341-3.
- [Google Scholar]
- Extracapillary glomerulonephritis and renal amyloidosis. Am J Kidney Dis. 1996;28:695-99.
- [Google Scholar]






