Translate this page into:
Acute Kidney Injury in Chronic Liver Disease in Northwest India: Still a Battle to Conquer
Corresponding author: Sanjeev Sharma, Department of Nephrology, SMS Medical College and Hospital, Jaipur, Rajasthan, India. E-mail: sanjeevkamal71@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Saxena D, Yadav M, Kumar T, Sharma S, Beniwal P, Malhotra V, et al. Acute Kidney Injury in Chronic Liver Disease in Northwest India: Still a Battle to Conquer. Indian J Nephrol. 2024;34:317-22. doi: 10.25259/ijn_286_23
Abstract
Background:
Patients with cirrhosis are susceptible to development of acute kidney injury (AKI), which leads to poor outcome. We conducted a study to evaluate the spectrum of AKI in patients with cirrhosis.
Materials and Methods:
This study was conducted in consecutive cirrhotic patients with AKI admitted in a tertiary care center of India from April 2020 to December 2022. Details including history, examination findings, and results of laboratory investigations were recorded.
Results:
A total of 243 patients were enrolled in this study. The majority (91.3%) of the patients were males. The most common etiology of cirrhosis was alcohol in 58.4% (n = 142) followed by hepatitis B in 10.3% (n = 25) of patients. Pre-renal form of AKI was present in 54.4% (n = 132) of patients and hepatorenal syndrome (HRS) in 21.8% (n = 53) of patients. IgA nephropathy was the commonest (n = 6) glomerular pathology in nonresponders with intrinsic renal disease. Majority of the patients belonged to stage II (46.9%) and stage I AKI (37%), while only 16.1% had stage III AKI. Various stages of AKI showed a significant correlation (P < 0.05) with Child–Turcotte–Pugh (CTP) score and Model for End-stage Liver Disease (MELD)-Na score. The overall in-hospital mortality rate was found to be 18.5% (n = 45).
Conclusion:
Renal dysfunction is a frequent complication among cirrhotic patients. Pre-renal factors were the most common cause of AKI in cirrhotics. Stages of AKI showed significant correlation with liver prognostic scores. Renal biopsy should be considered in patients not responding to treatment, to guide further management.
Keywords
Acute kidney injury
Biopsy
Cirrhosis
Ethanol
Glomerular
Introduction
Burden of liver cirrhosis is increasing globally as well as in Indian population.1 This surge is probably because of increasing incidence of nonalcoholic steatohepatitis (NASH) and alcohol abuse.1 Cirrhosis accounts for 1.16 million deaths per year worldwide, making it the 11th most common cause of death.2 India accounts for one-fifth (18.3%) of all cirrhosis deaths globally.3 Patients with cirrhosis are susceptible to renal dysfunction because of splanchnic vasodilation and substantial volume shift. In hospitalized patients, the prevalence of acute kidney injury (AKI) ranges from 20% to 50%.4-6 AKI can occur in these patients because of pre-renal insult, intrinsic renal disease, postrenal factors, or due to hepatorenal syndrome (HRS-AKI).7 Causes of intrinsic renal disease include acute tubular necrosis (ATN), glomerulopathies, tubulointerstitial pathologies, and vascular pathologies. Glomerular diseases commonly reported in cirrhotic patients include IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis (MPGN). The exact frequency of each cause in patients with cirrhosis has not been described yet. Identification of underlying etiology is important as treatment differs substantially. Pre-renal AKI (PRA) responds well to plasma volume expansion, whereas HRS-AKI and ATN require specific treatment approaches and are associated with high mortality and morbidity.7,8 The new criteria from the International Ascites Club provide a simple and prognostically relevant staging system for AKI in cirrhosis depending on the relative increases in serum creatinine.7,8 As per these criteria, AKI in cirrhosis is defined by an increase in serum creatinine of 0.3 mg/dl (26.4 μmol/l) in <48 h, or a 50% increase in serum creatinine from a baseline within ≤3 months. Furthermore, as per the International club of ascites ICA-AKI criteria, AKI has been classified into three stages (1–3) depending on the intensity of rise in serum creatinine.9,10 This staging system correlates well with the prognosis in patients with cirrhosis, with stages 2 and 3 having a poor outcome compared to stage 1.9,11 Majority of the studies have found pre-renal injury (70%) as the most common cause of AKI in cirrhotics, followed by intrinsic renal causes (30%) and postrenal factors (<1%).12,13 History of ongoing gastrointestinal losses like diarrhea/vomiting responsible for hypovolemia, treatment history, and history to rule out infections like cellulitis, etc., should be obtained. Depending upon the history and careful assessment of patients, further management should be planned to prevent morbidity and mortality. The present study was conducted to evaluate the risk factors and spectrum of AKI among patients with decompensated cirrhosis.
Materials and Methods
We conducted a prospective, observational study on cirrhotic patients with AKI admitted in a tertiary care center of India from April 2020 to December 2022. The study protocol was reviewed and approved from the SMS Medical College and attached Hospitals, Jaipur-302004. Rajasthan (India), with the approval number 376 MC/EC/2022. All cirrhotic patients with AKI admitted during the study period with age >18 years were included in this study. Patients with previously known renal disease, post-organ transplant patients, and patients with malignancy were excluded. A total of 368 patients were assessed for eligibility and out of them, 243 were included for the final analysis [Figure 1]. Demographic and clinical details of the patients, along with results of laboratory investigations were collected [Table 1]. Child–Turcotte–Pugh (CTP) score and Model for End-stage Liver Disease (MELD) score of patients were also noted. The study protocol was according to the ethical guidelines and was approved by the institutional committee.
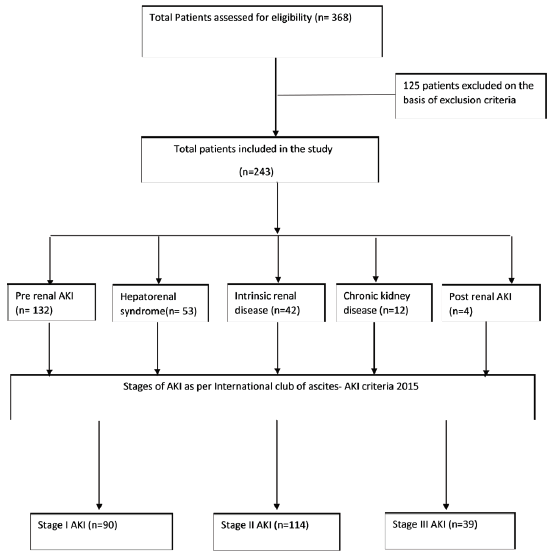
- Consort flow diagram of the study. AKI: Acute Kidney Injury
| Characteristics | Total number of patients (n = 243) |
|---|---|
| Age (years) | 47.85 ± 12.53 |
| Male | 222 (91.3%) |
| BMI (kg/m2) | 22.29 ± 1.84 |
| Mid arm circumference (cm) | 21.32 ± 1.38 |
| Hemoglobin (g/dl) | 8.10 ± 2.34 |
| Urea (mg/dl) | 89.67 ± 31.23 |
| Creatinine (mg/dl) | 2.59 ± 1.1 |
| Serum protein (g/dl) | 5.76 ± 1.09 |
| Serum albumin (g/dl) | 2.65 ± 0.42 |
| Bilirubin (mg/dl) | 7.34 ± 9.49 |
| Serum lactate (mmol/l) | 1.83 ± 1.32 |
| Serum sodium (mEq/l) | 131.87 ± 5.90 |
| Serum potassium (mEq/l) | 4.44 ± 0.88 |
| Urine sodium (mEq/l) | 54.79 ± 27.81 |
| Urine potassium (mEq/l) | 34 ± 14.18 |
| CTP score | 11.82 ± 2.37 |
| MELD-Na score | 30.19 ± 5.93 |
BMI: body mass index; CTP: Child Turcotte Pugh; MELD: Model for End-stage Liver Disease.
Definitions
Cirrhosis of liver: Cirrhosis of liver was diagnosed by a gastroenterologist depending on the clinical features, laboratory parameters, liver imaging, endoscopy, and liver biopsy (if available).
AKI: The new 2015 ICA-AKI criteria7 were used to diagnose AKI, which was categorized into three stages [Appendix 1].
Furthermore, patients with AKI were classified into PRA, HRS, postrenal AKI, and intrinsic renal disease (ATN/ glomerular disease).
PRA: Patients of cirrhosis with AKI secondary to hypovolemia due to gastrointestinal bleeding, aggressive diuretic treatment, or lactulose-induced diarrhea are said to have PRA.
HRS: Patients were classified as having HRS based on 2015 ICA criteria for AKI,7 which include the following: (1) presence of liver cirrhosis with ascites, (2) diagnosis of AKI based on the ICA-AKI criteria, (3) no improvement in serum creatinine after two consecutive days of plasma volume expansion with albumin 1 g/kg of body weight and withdrawal of diuretics, (4) absence of nephrotoxic drugs, (5) absence of shock, and (6) no macroscopic evidence of structural kidney damage, that is, absence of proteinuria (<500 mg/day), absence of microhematuria (<50 red blood cells [RBCs]/high-power field), and normal findings on ultrasonography.
Intrinsic renal disease: Patients with intrinsic renal disease comprising glomerular disease and ATN were diagnosed after excluding PRA, HRS, and postobstructive cause of AKI. Diagnosis of AKI was reviewed and confirmed by nephrologists independently. Renal biopsies were performed in patients with active urinary sediments and nonresponders.
Statistical analysis
Normally distributed continuous variables were expressed as means and standard deviation, while non-normally distributed continuous variables were reported as median and interquartile range. Categorical variables were expressed as percentages. Significant parameters associated with the renal dysfunction were analyzed using binary logistic regression analysis. A P-value of less than 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS) 21 software was used for statistical analysis.
Results
A total of 243 patients were included, with 91.3% (n = 222) males, anda mean age of 47.85 years (±12.54). The most common etiology of cirrhosis was ethanol (58.4%), followed by chronic hepatitis B (10.3%) [Table 2].
| Etiology of cirrhosis | Number (%) |
|---|---|
| Ethanol | 142 (58.4%) |
| Hepatitis B | 25 (10.3%) |
| Cryptogenic | 23 (9.6%) |
| Ethanol + NASH | 20 (8.2%) |
| NASH | 18 (7.4%) |
| Ethanol + hepatitis B | 6 (2.5%) |
| NASH + hepatitis B | 3 (1.2%) |
| Hepatitis C | 3 (1.2%) |
| Autoimmune hepatitis | 2 (0.8%) |
| Wilson disease | 1 (0.4%) |
NASH: nonalcoholic steatohepatitis.
The most common type of AKI was found to be PRA in 54.4% of patients, followed by HRS in 21.8%. Postrenal factors were bilateral ureteric obstruction in three patients and neurogenic bladder in one patient. Distribution of different types of AKI in this study is shown in Table 3 and Figure 2.
| Type of AKI | Number (%) |
|---|---|
| Pre-renal AKI | 132 (54.4%) |
| Hepatorenal syndrome | 53 (21.8%) |
| Intrinsic renal disease | 42 (17.3%) |
| Chronic kidney disease | 12 (4.9%) |
| Postrenal AKI | 4 (1.6%) |
AKI: acute kidney injury.
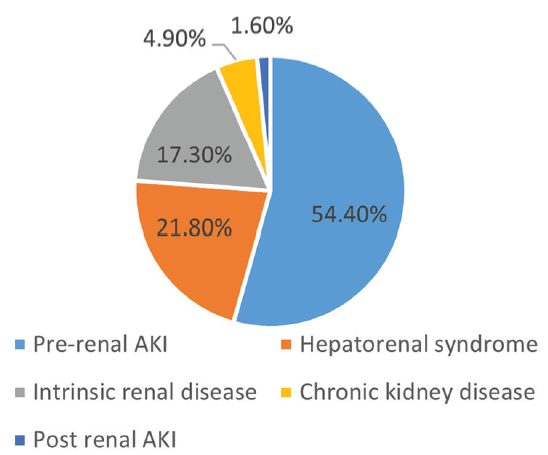
- Etiological classification of cirrhosis. AKI: Acute kidney injury.
Out of 42 patients with intrinsic renal disease, 26 patients recovered with conservative treatment and percutaneous renal biopsy was performed in 11 patients. Out of 11 patients [Figure 3], two patients were found to have severe ATN, two patients with hepatitis B had MPGN [Figure 4], and 1 had membranous nephropathy. The most common type of glomerular lesion was IgA nephropathy [Figure 5] in six patients. Renal biopsy could not be performed in rest of the nonresponders because of various contraindications.
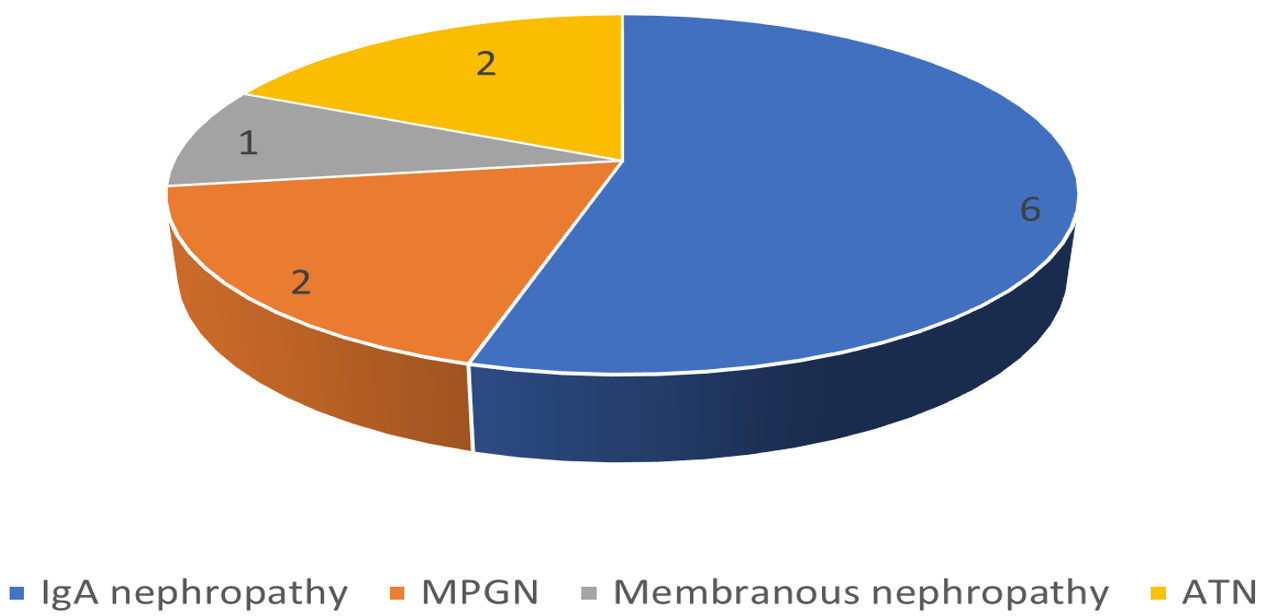
- Histopathologic findings on renal biopsy of nonresponders. ATN: Acute tubular necrosis; MPGN: Membranoproliferative glomerulonephritis; IgA: Immunoglobulin A.
- Membranoproliferative glomerulonephritis (periodic acid–Schiff stain, magnification ×400).
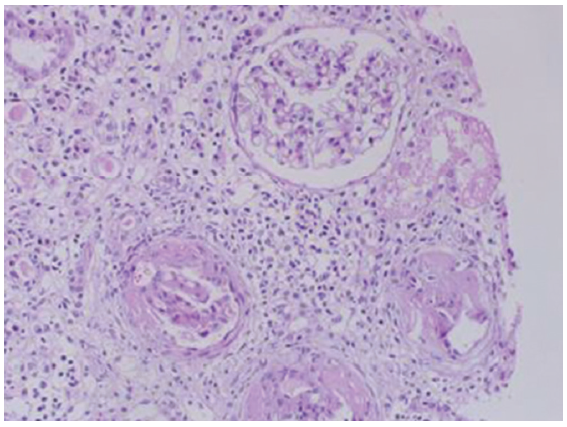
- IgA nephropathy (hematoxylin and eosin stain, magnification ×100).
Majority of the patients had stage II (46.9%) and stage I (37%) AKI, while only 16.1% of the patients were in stage III AKI. Table 3 shows the comparison of general characteristics, laboratory parameters, and outcome of patients with cirrhosis with different stages of AKI.
Mean values of total leukocyte count and serum creatinine were significantly different in various stages of AKI (P < 0.05). Stages of AKI showed a significant association (P < 0.05) with CTP score and MELD-Na score [Table 4].
| Parameters | Severity of AKI | P | ||
|---|---|---|---|---|
| Stage I ( n = 90) | Stage II (n = 114) | Stage III (n = 39) | ||
| Age (years) | 50.06 ± 12.54 | 47.71 ± 13.55 | 48.15 ± 7.04 | 0.38 |
| BMI (kg/m2) | 22.09 ± 2.05 | 22.22 ± 1.65 | 22.06 ± 1.75 | 0.83 |
| MAC (cm) | 21.52 ± 1.84 | 21.40 ± 1.96 | 21.80 ± 0.99 | 0.48 |
| Hb (g/dl) | 8.04 ± 2.35 | 8.39 ± 2.40 | 7.36 ± 2.06 | 0.05 |
| Platelet count (×1000/mm3) | 88.40 ± 53.24 | 97.39 ± 47.59 | 91.07 ± 55.25 | 0.44 |
| TLC (×1000/mm3) | 7.83 ± 3.03 | 11.26 ± 7.49 | 13.99 ± 8.36 | <0.01 |
| Serum creatinine (mg/dl) | 1.82 ± 0.20 | 2.53 ± 0.43 | 4.54 ± 1.36 | <0.01 |
| Serum lactate (mmol/l) | 1.65 ± 1.02 | 1.78 ± 0.89 | 1.67 ± 0.43 | 0.55 |
| Serum sodium (mEq/l) | 132.16 ± 5.11 | 131.60 ± 5.82 | 132 ± 7.88 | 0.79 |
| Serum potassium (mEq/l) | 4.43 ± 0.94 | 4.43 ± 0.84 | 4.50 ± 0.86 | 0.90 |
| Serum albumin (g/dl) | 2.65 ± 0.46 | 2.64 ± 0.38 | 2.60 ± 0.42 | 0.99 |
| Serum AST (IU/l)b | 69 (11–320) | 91.5 (16–1150) | 65 (30–179) | 0.97 |
| Serum ALT (IU/l)b | 34.5 (10–410) | 38 (12–220) | 25 (12–66) | <0.01 |
| Serum bilirubin (mg/dl)b | 2.75 (0.4–21) | 3.35 (0.5–35) | 3.3 (0.9–44) | <0.01 |
| Serum cholesterol (mg/dl) | 107.04 ± 47.45 | 108.15 ± 44.51 | 106.46 ± 47.82 | 0.38 |
| CTP score | 11.02 ± 2.35 | 11.88 ± 2.37 | 12.58 ± 2.40 | 0.83 |
| MELD-Na score | 26.70 ± 4.53 | 31.63 ± 5.81 | 34.07 ± 5.04 | 0.48 |
Sixty-four patients (26.33%) required renal replacement therapy. Thirty-five patients received one or more sessions of intermittent hemodialysis, 22 patients underwent acute peritoneal dialysis, and sustained low efficiency dialysis was done in seven patients. The overall in-hospital mortality in our study population was 18.5% (n = 45).
Discussion
Renal dysfunction, especially HRS, has an unfavourable prognosis in patients with cirrhosis. Renal insufficiency is often associated with other accompanying cirrhosis complications like hepatic encephalopathy, variceal bleed, ascites, etc.14 The first description of renal dysfunction in the setting of cirrhosis was from Europe and the United States in late 19th century.15 Hecker and Sherlock16 in 1956 gave the first detailed description of HRS. In the past, most of the clinical studies focused their attention on HRS, which is generally seen in advanced stages of cirrhosis.
In recent years, better understanding of pathophysiology of AKI has led to changes in diagnostic criteria, with specific treatment approaches responsible for improved outcome.17
Demographic parameters of patient population included in our study are in accordance with the previous studies from India. In studies conducted by Sethuraman and Balasubramanian18 and Rathi et al.,19 the mean age of the patients was 49.58 and 49.5 years, respectively. Mean age of the patients in our study was 47.85 years (±12.54). Majority of patients in our study were male, which is similar to previously conducted studies.18,19 In the present study, the most common etiology of cirrhosis was found to be ethanol followed by hepatitis B, which was replicated previously by a study from our center and other part of the country.20,21 Similar results were replicated by a cohort study in Swedish population, with higher incidence of cirrhosis in men compared to women, and also, ethanol was the main culprit for cirrhosis (50.5%).22
The spectrum of renal dysfunction in our study was prerenal AKI in 54.4%, HRS in 21.8%, intrinsic renal disease in 17.3%, chronic kidney disease in 4.9%, and postrenal AKI in 1.6% of patients. Similar spectrum of renal dysfunction was seen in studies conducted by Arora et al.,23 Keshav and Kumar,24 and Soni and Nagpal.25
In patients with intrinsic renal disease with persistent renal dysfunction, the most common glomerular pathology was IgA nephropathy. In a study of liver transplant candidates with renal failure of undetermined etiology, the most common glomerular lesion on renal biopsy was found to be IgA nephropathy (45%).26 In another study with 65 patients, the most common glomerular pathology was IgA nephropathy in 27 (41.5%) patients, followed by MPGN in 11 patients (16.9%).27
Majority of the patients in our study had stage II and stage I AKI as per the ICA-AKI criteria.7 In a study from northern India, patients in stage I and stage II AKI constituted 77.4% and 19.7%, respectively.17 Another study by Piano et al.28 revealed that most of the patients had stage I AKI (52.5%) followed by stage III (31.2%).
Stages of AKI significantly correlated with the CTP and MELD-Na scores in our study; similar correlation was found with total leukocyte count. Identical results were replicated in a study conducted by Khatua et al.29 and Shetty et al.30 Overall in-hospital mortality in our study population was 18.5%; similarly, in the study by Khatua et al.,29 mortality in hospitalized patients was 17.8%. But higher mortality rates were seen in the studies conducted by Shetty et al. (44.7%)30 and de Carvalho et al. (52.7%).10
Our study results should be interpreted keeping in mind the limitations. This was a single-center study, and findings may not be generalizable to whole populations. However, no study has been published recently using the new 2015 Ascites Club Criteria in our population. Thus, we believe that results from the current study represent an important contribution on AKI patients with cirrhosis.
Conclusion
Renal dysfunction is a frequent cause of morbidity and mortality among cirrhotic patients. It was observed that PRA was the most common type of renal dysfunction. Patients with worst liver disease were found to have severe AKI with stage II and III. Authors suggest that screening of AKI should be done in all patients with decompensated cirrhosis and renal biopsy should be considered in patients not responding to treatment, for timely diagnosis and appropriate management.
Conflicts of interest
There are no conflicts of interest.
References
- Global Epidemiology of chronic liver disease. Clin Liver Dis (Hoboken). 2021;17:365-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Burden of liver diseases in the world. J Hepatol. 2019;70:151-71.
- [CrossRef] [PubMed] [Google Scholar]
- Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014;12:145.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Relevance of new definitions to incidence and prognosis of acute kidney injury in hospitalized patients with cirrhosis: A retrospective population-based cohort study. PLoS One. 2016;11:e0160394. doi: 10.1371/journal.pone.0160394
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury in acute-on-chronic liver failure: Where does hepatorenal syndrome fit? Kidney Int. 2017;92:1058-70.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of acute kidney INJURY in patients with cirrhosis: Revised consensus recommendations of the international club of ascites. J Hepatol. 2015;62:968-74.
- [CrossRef] [PubMed] [Google Scholar]
- Tubular site of renal sodium retention in ascitic liver cirrhosis Evaluated by lithium clearance. Eur J Clin Invest. 1990;20:111-7.
- [CrossRef] [PubMed] [Google Scholar]
- Renal failure and cirrhosis: A systematic review of mortality and prognosis. J Hepatol. 2012;56:810-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol. 2012;46:e21-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury and acute on chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616-22.
- [CrossRef] [PubMed] [Google Scholar]
- Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Advances in the diagnosis and treatment of acute kidney injury in cirrhosis patients. BioMed Res Int. 2017;2017:8523649. doi: 10.1155/2017/8523649
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence, predictive factors, and prognosis of hepatorenal syndrome in cirrhosis. Gastroenterology. 1993;105:229-36.
- [CrossRef] [PubMed] [Google Scholar]
- Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956;268:1121-5.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury in liver cirrhosis: New definition and application. Clin Mol Hepatol. 2016;22:415-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical spectrum of precipitating factors of hepatic encephalopathy in cirrhosis of liver and its relation to prognosis in a tertiary care hospital – a retrospective study. Int J Contemp Med Surg Radiol. 2019;4:B65-70.
- [Google Scholar]
- Prevalence of minimal hepatic encephalopathy in patients with liver cirrhosis: A cross-sectional, clinic epidemiological, multicentre nationwide study in India: The PREDICT study. J Clin Exp Hepatol. 2019;9:476-83.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Spectrum of chronic liver disease admitted to a medical college hospital in Northern India: Is there cause for concern? Indian J Gastroenterol. 2014;33:480-1.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence, aetiology and related comorbidities of cirrhosis: A swedish population-based cohort study. BMC Gastroenterol. 2020;20:84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Profile of Acute kidney injury in patients with decompensated cirrhosis at a tertiary-care center in uttarakhand, India. Dig Dis Basel Switz. 2020;38:335-43.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of renal dysfunction in cirrhotic patients of Jharkhand. Indian J Appl Res. 2018;8:48-50.
- [Google Scholar]
- Spectrum of renal disorders in patients with liver cirrhosis: How grievous is it? Int J Contemp Med Res. 2020;7:I1-5.
- [Google Scholar]
- Renal function outcomes following liver transplantation and combined liver-kidney transplantation. Nat Clin Pract Nephrol. 2007;3:507-14.
- [CrossRef] [PubMed] [Google Scholar]
- The Spectrum of renal lesions in patients with cirrhosis: A clinicopathological study. Liver Int. 2010;30:725-32.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the acute kidney injury network CRITERIA in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury in hospitalized cirrhotic patients: Risk factors, type of kidney injury, and survival. JGH Open. 2020;5:199-206.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acute kidney injury in patients with cirrhosis of liver: Clinical profile and predictors of outcome. Indian J Gastroenterol. 2018;37:248-54.
- [CrossRef] [PubMed] [Google Scholar]







