Translate this page into:
Nephrotic Syndrome with Acute Kidney Injury after Covaxin (BBV152) COVID-19 Vaccination: A Case from India
Address for correspondence: Dr. Prem S. Patel, Department of Nephrology, Indira Gandhi Institute of Medical Science, Patna - 800 014, Bihar, India. E-mail: drpspdm@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
Vaccination with coronavirus disease 2019 (COVID-19) vaccines has succeeded in minimizing the morbidity and mortality from COVID-19. These vaccines claim an excellent short-term safety profile.[1] Long-term safety profile of these vaccines still remains to be elucidated. Here, we are reporting a case of podocytopathy leading to nephrotic syndrome with acute kidney injury (AKI) after the first dose of Covaxin BBV152 vaccination. Covaxin is India’s indigenous BBV152 vaccine developed by Bharat Biotech Limited in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV) and approved by the World Health Organization (WHO) for emergency use against COVID-19 on November 3, 2021.[2]
A 16-year-old young male presented with generalized swelling 4 days after the first dose of Covaxin BBV152 vaccination on January 4, 2022. Four days later, he noticed swelling over the face, which progressed over 2–3 weeks and involved the entire body. It was not associated with fever, skin rash, joint pain, jaundice, hematuria, and oliguria. There was no history of similar edematous illness in the past. Other medical, surgical, treatment, and drug histories were insignificant. General examination was nonrevealing, except anasarca. His blood pressure was 120/76 mmHg, pulse rate 80/min, respiratory rate 18/min, and body temperature was 98.6°F. Systemic examinations were unremarkable. He was given symptomatic treatment with torsemide 10 mg twice daily by a physician at a local center. His edema persisted and urinary examination showed proteinuria, and hence, he was referred to us after about 3 months of treatment. On evaluation, urine examination revealed the following: albumin 3+, red blood cells (RBCs) 2–3/hpf, and pus cells 3–5/hpf. Also, the 24-h urinary protein was 4.1 g. Other parameters are summarized in Table 1. His chest X-ray, electrocardiogram (ECG), and 2D echocardiography were normal. COVID-19 real-time polymerase chain reaction (RT-PCR) from nasopharyngeal swab was negative.
| Parameters | Result |
|---|---|
| 1. Hematology | |
| Total leukocyte count | 6980/mm |
| Differential leukocyte count | N65%/L20% |
| Platelet count | 301,000/mm3 |
| Hemoglobin | 15.2 g/dl |
| ESR | 18 mm/h |
| 2. Biochemistry and serology | |
| Serum creatinine | 1.73 mg/dl |
| Blood urea | 50 mg/dl |
| Na+/k+ | 132/3.8 mmol/dl |
| Ca2+/P | 8.3/5.4 mg/dl |
| Serum bilirubin | 0.4 mg/dl |
| ALP | 189 U/l |
| SGPT/SGOT | 19/14 IU/l |
| Total serum protein | 6.4 g/dl |
| Serum albumin | 2.1 g/dl |
| Total cholesterol | 331 mg/dl |
| HDL | 41 mg/dl |
| LDL | 182 mg/dl |
| TG | 249 mg/dl |
| VLDL | 50 mg/dl |
| RBS | 98 mg/dl |
| HIV/HBsAg/HCV | Nonreactive |
| Urine analysis | Albumin 3+, RBCs 2-3/hpf, pus cells 3-5/hpf |
| 24-h urinary protein | 4.1 g |
| ANA | Negative |
| C3 and C4 | 117 and 28.8 mg/dl, respectively |
| 3. Radiology | |
| ECG | Normal sinus rhythm |
| 2D ECHO | Normal study |
| USG abdomen | Bilateral normal-sized kidney, mild ascites |
ALP=alkaline phosphatase, ANA=antinuclear antibody, ECG=electrocardiogram, ECHO=echocardiography, ESR=erythrocyte sedimentation rate, HBsAg=hepatitis B surface antigen, HCV=hepatitis C virus, HDL=high-density lipoprotein, HIV=human immunodeficiency virus, hpf=high-power field, LDL=low-density lipoprotein, RBC=red blood cell, RBS=random blood sugar, SGOT=serum glutamate oxaloacetate transaminase, SGPT=serum glutamate pyruvate transaminase, TG=triglyceride, USG=ultrasonogram, VLDL=very low-density lipoprotein
After giving written consent, he underwent kidney biopsy on July 13, 2022. Renal histopathology revealed a total of 11 glomeruli, none globally sclerosed. The glomeruli revealed focal dilatation and congestion of capillary lumina and non-proliferative morphology. There was no evidence of segmental sclerosis, tuft necrosis, subendothelial/congophilic deposits, or crescent formation. Tubular atrophy and interstitial fibrosis involved less than 10% of sampled cortex. Tubules showed focally prominent cytoplasmic vacuolar change. Interstitial inflammation was insignificant. Arteries and arterioles sampled appeared unremarkable. Immunofluorescence microscopy was unremarkable. Ultrastructure examination revealed widespread effacement (about 70%) of visceral epithelial cell foot processes. Electron-dense or organized deposits were not identified in glomerular basement membrane (GBM) or mesangium [Figure 1a and b]. He has been advised oral prednisolone 1 mg/kg/day along with supportive treatment with diuretics (torsemide 10 mg twice a day) and renin angiotensin system inhibitor (ramipril 2.5 mg once a day). After 4 weeks, he is now in complete remission and shows 24-h urinary protein of 230 mg.
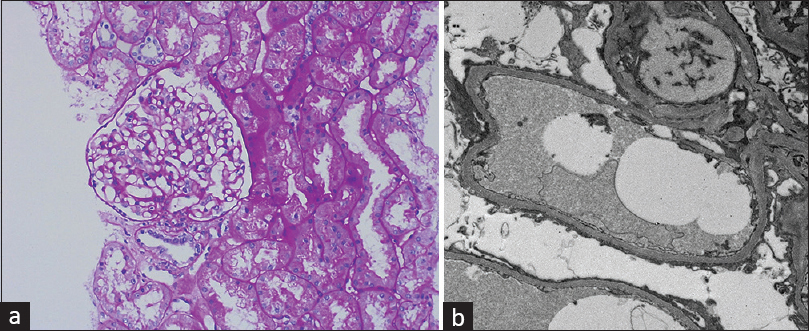
- (a) On light microscopy, the glomeruli show focal dilatation and congestion of capillary lumina and non-proliferative morphology. (Periodic acid–Schiff PAS, 100×). (b) Electron microscopy (EM) shows widespread effacement (about 70%) of visceral epithelial cell foot processes (Electron microscopy EM, 2000×)
The first case of kidney involvement after administration of COVID-19 vaccine was published by Lebedev et al.[3] Subsequently, many case reports of nephrotic syndrome and glomerulonephritis have been reported. [45] Minimal change disease is the most frequently reported podocytopathy after COVID-19 vaccination. However, focal segmental glomerulosclerosis (FSGS) and collapsing glomerulopathy also have been seen.[5] Podocyte injury is the central step leading to diffuse effacement of foot process in podocytopathies and morphologically manifests as either minimal change disease (MCD) or FSGS. Recent evidence points toward association of COVID-19 vaccine with podocytopathy.[4] However, the exact pathophysiology of COVID-19 vaccine-associated nephrotic syndrome remains to be elucidated. Different COVID-19 vaccines use different mechanisms to elicit immune responses in the host. Inactivated whole virion is used in Covaxin (BBV152) vaccine.[1] Two different hypotheses have been proposed for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine-induced kidney diseases. One hypothesis proposes that dysregulated T-cell activation and production of circulating permeability factor led to diffuse effacement of podocyte foot process in MCD.[5] Another hypothesis proposes that vaccine-induced development of autoimmunity through molecular mimicry and cross-reactivity causes glomerulonephritis after SARS-CoV-2 vaccination. Thus, COVID-19 vaccine-mediated T-cell dysfunction and development of autoimmunity could be the underlying immune mechanism resulting in nephrotic syndrome and glomerulonephritis after SARS-CoV-2 vaccination. Nephrotic syndrome has been described after the use of the different types of COVID-19 vaccine. [45] Majority of the podocytopathy and glomerulonephritis cases have been reported with ChAdOx1 nCoV-19 and mRNA vaccines.[5] This is probably the first report of podocytopathy (likely minimal changes disease) leading to nephrotic syndrome with acute kidney injury after administration of Covaxin vaccine. The temporal association between vaccine exposure and the onset of disease and the absence of any other triggering factor in our case support a causal link; however, this is difficult to prove.
To conclude, evidence suggests the occurrence of podocytopathy and glomerulopathies after COVID-19 vaccination. Thus, kidney biopsy is advisable for whoever develops proteinuria and edema after administration of the COVID-19 vaccine. It is not clear whether administration of second dose of the vaccine is safe or not.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to Dr. Alok Sharma, MD Pathology, Technical Director – Renal Pathology and Transmission Electron Microscopy, for providing the light microscopy and electron microscopy images of renal histology.
References
- Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152):Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173-84.
- [Google Scholar]
- 2022. Who.int. Available from:https://www.who.int/news/item/03-11-2021-who-issues-emergency-use-listing-for-eighth-covid-19-vaccine
- Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:142-5.
- [Google Scholar]
- New-onset and relapse of nephrotic syndrome following COVID-19 vaccination:A questionnaire survey in Japan. Clin Exp Nephrol 2022:1-8. doi:10.1007/s10157-022-02231-y
- [Google Scholar]






