Translate this page into:
Acute kidney injury in patients with human immunodeficiency virus infection
Address for correspondence: Dr. Jai Prakash, Department of Nephrology, Institute of Medical Sciences, Banaras Hindu University, Varanasi - 221 005, Uttar Pradesh, India. E-mail: jpojha555@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute kidney injury (AKI) is an important cause of hospitalization and morbidity in human immunodeficiency virus (HIV)-positive patients. However, the data on AKI in such patients is limited. The aim of the present study was to analyze the incidence, causes and outcome of AKI in HIV-positive patients from our antiretroviral therapy centre. All HIV-positive patients were evaluated for evidence of clinical AKI. AKI was noted in 138/3540 (3.9%) patients. Of 138 AKI patients, 96 (69.6%) had acquired immuno deficiency syndrome and 42 (30.4%) were HIV seropositive. Majority of AKI patients belonged to AKI network (AKIN) Stage II (42%) or III (48.5%) at presentation. Prerenal, intrinsic and postrenal AKI were noted in 53.6%, 44.2% and 2.2% of cases, respectively. Hypovolemia (44.2%) and sepsis (14.5%) contributed to AKI in vast majority of cases. AKI was multifactorial (volume depletion, sepsis and drugs) in 39% of patients. Acute tubular necrosis (ATN) was the most common intrinsic lesion. Acute interstitial nephritis and diffuse endocapillary proliferative glomerulonephritis were noted in five and two cases, respectively. In-hospital mortality was 24.64%. Lower CD4 count, decreased serum albumin level and Stage 4 WHO disease were associated with higher mortality. At 3 months or more follow-up complete recovery of renal function, chronic kidney disease Stage 3-5 and progression to end stage renal disease were noted in 58.69%, 14.5% and 2.2% of cases, respectively. Thus, prerenal factors and ischemic ATN were the most common cause of AKI in HIV-infected patients. Recovery of renal function was seen in 59% of cases, but AKI had high in-hospital mortality.
Keywords
Acute glomerulonephritis
acute kidney injury
acute tubular necrosis
human immunodeficiency virus
sepsis
Introduction
The epidemic of acquired immunodeficiency syndrome (AIDS) is a global problem.[1] With the introduction of highly active antiretroviral therapy (HAART), morbidity and mortality due to AIDS have declined rapidly.[2] However, associated co-morbid conditions can reduce survival in these patients.[23] Renal dysfunction, especially acute kidney injury (AKI), is an important cause of hospitalization and mortality in human immunodeficiency virus (HIV)-positive patients.[4567] AKI in HIV patients is well studied in developed world and available literature suggest incidence to be 2.7-5.9/100 person-years.[8910] However, there is paucity of data on clinical characteristics of AKI in HIV-positive patients from our country. The aim of this prospective study was to analyze the incidence, causes and outcome of AKI in HIV-infected patients from our centre.
Materials and Methods
Study design
This prospective study includes all adult HIV sero-positive patients of 18 years or above attending ART Center of Institute of Medical Sciences, Banaras Hindu University, Varanasi, UP, India, between August 1, 2010 and July 31, 2013. National AIDS Control Organization (NACO) 2007 guideline was used for the diagnosis of AIDS and HIV infection in our study. The patients included in this study were screened for evidence of AKI with help of relevant history, physical examination and laboratory investigations. Urinalysis was carried out in all patients. Serum lactate dehydrogenase, serum glutamic oxaloacetic transaminase, serum glutamic-pyruvic transaminase, platelet count and coagulation profile were carried out as and when required. Biochemical investigations included estimation of urea, creatinine, sodium, potassium, chloride, calcium, phorphorus, alkaline phosphatase, total protein, and albumin. AKI were categorized into prerenal, renal and postrenal group based on clinical and laboratory parameters. Associated comorbidities such as diabetes, hypertension, malignancy, liver disease and hepatitis B and C viral serology were recorded. Patients with preexisting chronic kidney disease (CKD) and hospital acquired AKI were excluded. The need of renal replacement therapy, in-hospital mortality and renal function status at discharge were recorded in all patients. The status of renal functions using urinalysis, serum creatinine level and ultrasonography of kidney was evaluated at 3 months or more after episode of AKI in all survivors to define CKD or progression to end stage renal disease (ESRD). The following definitions were used in this study.
AKI was defined as per AKIN criteria, that is, an increase in serum creatinine of ≥0.3 mg/dl or ≥50% developing over <48 h and/or urine output of <0.5 ml/kg/h for >6 h.[11] Prerenal AKI was considered when there was clinical and/or laboratory evidence of volume loss or reduced renal blood flow, and renal functions improved with hydration.[5] Diagnosis of acute tubular necrosis (ATN) was based on clinical settings of patients with AKI (volume depletion, shock and sepsis). Intrinsic AKI were classified according to the primary site of kidney injury on biopsy. Postrenal AKI was considered in the presence of obstruction of urinary tract and reversal of renal function on release of obstruction. Based on renal function status at 3 months or more after AKI episode, the survivors were grouped as: (1) fully recovered when serum creatinine level is below 1.5 mg/dl, (2) CKD when serum creatinine is above 1.5 mg/dl[12] and (3) ESRD as per Kidney Disease Improving Global Outcomes 2012 guideline.[13] Sepsis was defined as a systemic inflammatory response to a documented infection. The severity of sepsis was classified as per American College of Chest Physicians/Society of Critical Care Medicine consensus conference.[14]
Statistical analysis
Categorical data were expressed in numbers and continuous variables as mean ± standard deviation. Chi-square test and/or Fisher's tests were used to analyze categorical data, while continuous data were analyzed using unpaired t-test. P < 0.05 was considered as point of statistical significance.
Results
During the study period, total 3540 (male/female ratio: 2014/1526) patients were enrolled at our ART center. 2194/3540 patients (61.98%) were prescribed ART as they fulfilled NACO guideline criteria for initiation of ART.[15] The clinical evidence of renal disease was noted in 340 (9.6%) patients and 138 (3.9%) cases had features of AKI. Of 138 AKI patients, 96 (69.6%) had AIDS and 42 (30.4%) cases were HIV sero-positive. The mean age of patients was 35.33 ± 9.76 years and majority of patients were in the age range between 30 and 49 years. Baseline clinical characteristics of patients are shown in Table 1. Ninety percent of patients with AKI fell into AKIN Stage II (42%) and AKIN Stage III (48.5%) category [Table 2]. Males dominated the study population (76.08%, n = 105). Most of the patients were anemic (n = 117, 84.78%, as per WHO criteria) with mean hemoglobin value of 9.25 ± 2.53 g/dl. Majority of cases were in advance stages of HIV infection with mean CD4 count of 201.38 ± 151.22 cell/μl and 85 (61.59%) patients had CD4 count <200 cell/μl. Blood urea, serum creatinine and serum albumin level were 110 ± 40.947 mg/dl, 5.36 ± 3.67 mg/dl and 2.92 ± 0.862 g/dl, respectively [Table 1].


Clinical feature of acute kidney injury
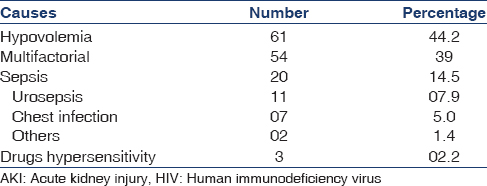
Prerenal AKI was noted in more than half of cases (n = 74; 53.62%). Hypovolemia and sepsis were the possible causes of AKI in 42.2% and 14.5% of cases, respectively. The multiple (hypotension, dehydration, sepsis and drugs) etiologies contributed to AKI in 54 (39%) of cases [Table 3]. Postrenal AKI was due to prostatic hypertrophy and bilateral ureteral obstruction (calculus) in two patients and one patient, respectively. Five patients with AKI had feature of acute interstitial nephritis (AIN) (infection related three and nonsteroidal antiinflammatory drug induced two). Aminoglycosides and contrast agent induced AKI were noted in two cases and one cases, respectively.
Renal histology
Renal biopsy was done in 12 cases because of prolonged course of AKI (>4 weeks). ATN, AIN and diffuse endocapillary proliferative glomerulonephritis (GN) were seen in five, two and two cases, respectively. We observed chronic interstitial nephritis, thrombotic micro-angiopathy and focal segmental GN in one case each.
Patients and renal outcome
Thirty-four (24.64%) patients succumbed to illness during course of hospital stay. Severe sepsis was the leading cause of death in 18 (53%) patients. The other causes of death were pneumocystis pneumonia in 4 (11.7%) cases, complicated malaria in 3 (8.8%) cases and disseminated tuberculosis in 3 (8.8%) cases. Cryptococcus meningitis, hepatic failure and hypovolemic shock were responsible for death in 2 (5.8%) patients each. The renal outcome of 104 patients, at the end of 3 months, after was (1) 81 (58.69%) patients had complete recovery of renal function; (2) 20 (14.5%) cases developed Stage 3-5 CKD and (3) 3 (2.2%) patients progressed to ESRD.
Clinical and laboratory parameters of acute kidney injury cases
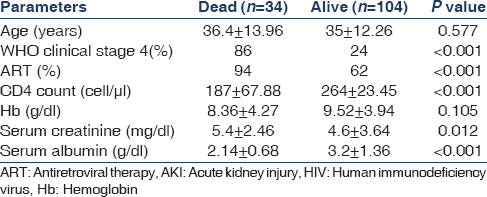
Clinical data of patients (living vs. dead) are shown in Table 4. Mean age was similar in both groups. CD4 count was significantly lower in mortality group in comparison to living patients (187.67 ± 67.88 vs. 264.27 ± 23.45, P < 0.001). About 94% patients were on ART in the mortality group, while only 62% patients received ART in the alive group (P < 0.001). Most patients (86%) in the mortality group had WHO clinical Stage 4, while only 24% patients in the alive group had Stage 4 disease (P < 0.001). The patients with mortality group had statistically significant low mean level of serum albumin (2.14 ± 0.68 vs. 3.2 ± 1.36 g/dl; P < 0.001). No statistically significant differences were observed in term of sex, age, mean hemoglobin, blood urea, calcium, phosphorus, sodium and potassium levels in either group.
Discussion
As of 2012, there were 35.3 million people worldwide living with HIV/AIDS, with 2.3 million new HIV infections per year and 1.6 million annual deaths due to AIDS.[16] The total number of people living with HIV/AIDS in India is estimated at 2.1 million in 2012, which equates to a prevalence of 0.3%.[17] In this study, we analyzed clinical characteristics of AKI in patients with HIV attending ART centre of our hospital. Most of the patients were of younger age group and 88/128 (63.8%) cases were male. Male predominance was expected since HIV infection in India is predominantly a disease of sexually active male gender.[17] However, female has less access to health resources because of ignorance, social stigmas and/or poor financial support.
Incidence of community-acquired AKI in the general population is around 1% among all hospitalizations, with overall good prognosis.[18] We observed incidence of community-acquired AKI in 3.9% of HIV-infected patients attending our hospital, which is comparable to studies reported from Western countries.[9] In a prospective study, Franceschini et al. observed 111 AKI episodes in 71 subjects in cohorts of 754 patients, with incidence rate of 5.9 episodes/100 person years.[9] Among hospitalized patients, recent study by Lopes et al. reported 18% incidence of AKI in HIV-positive patients.[4] Similar high rates of AKI have been observed in other studies in HAART era.[810]
Of all cases, 40% patients had advanced HIV infection and 61.6% had CD4 count of <200/μl. AIDS-related opportunistic infections were seen in 84.05%. With the introduction of HAART, AIDS-related opportunistic infections are showing a declining trend, while the presence of comorbid condition is assuming greater significance.[8919202122] However in our study, presence of comorbidities was seen in few patients only and most of the AKI cases occurred in the presence of AIDS-related opportunistic infections. Among various comorbid conditions, hepatitis C virus (HCV) coinfection is increasingly being considered as an important risk factor for AKI.[8910] HCV coinfection is observed in 15-30% of all HIV-positive patients.[23] Gastrointestinal bleeding, large volume paracentesis, overzealous use of diuretics with consequent reduced renal perfusion, sepsis especially spontaneous bacterial peritonitis, nephrotoxic drug administration or hepato-renal syndrome are important causes of AKI in HCV coinfection.[81020] In a study by Franceschini et al. 20% causes of AKI in HIV sero-positive patients were directly related to hepatic causes.[9] We did not observe HCV coinfection in our study. Similarly, preexisting CKD, diabetes, hypertension and active malignancy are other important comorbidities associated with increased risk of AKI in HIV-infected patients.[4810] AKI in HIV-infected patient is divided in two groups: “early onset”: Occurring within 3 months of HIV care; and “late onset”, which occurred after 3 months of HIV care.[10] AIDS-related opportunistic infections play an important role in the development of “early onset AKI” while associated comorbidities and HAART toxicity are important factors leading to “late onset AKI”.[10]
The most common AKI was prerenal (53.6%) in our study. Prerenal AKI, caused by diarrhea, vomiting, liver failure and infections, was the most common form of AKI in HIV patients, similar to our study.[9] A recent study from South Africa reported sepsis (60%), volume depletion and hemodynamic instability (19%), toxins (9%), urological obstruction (7%) and miscellaneous causes in 14% of cases causing AKI in HIV-positive patients.[24] Hypovolemia (44.2%) and sepsis (14.5%) were contributory factors for AKI in the vast majority of our patients. In addition, AKI was multifactorial in 39% of the cases. Lopes et al. observed AKI was multifactorial in 48.9% of HIV patients in their series.[4] Thus, our observations were in accordance with other reported studies.[4910] Nephrotoxic drugs are the important contributory factors leading to AKI in HIV-infected persons. The incidence of drug-related AKI in such cases ranges from 2% to 37.5%.[424] Nephrotoxic antibiotics, ART drugs and contrast agents are reported to cause AKI in HIV patients.[49] ART drugs like indinavir and nevirapine can cause acute crystalline nephropathy, AIN and ATN.[2526] We observed drug-related AKI in three cases. However HAART-related AKI was not seen in our study. Renal biopsy in 12 cases of AKI revealed ATN in 41% of patients and one case had biopsy features of thrombotic microangiopathy (TMA) in the present study. Peraldi et al. reported hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura in 32/92 (35%) and ATN in 24/92 (26%) of the cases.[27] TMA in HIV patients could be related to HIV infection itself; however, its incidence is uncommon.[9] Glomerular disease associated with HIV infection can cause AKI. Antiglomerular basement membrane antibody mediated cresentic GN causing AKI is reported in HIV patients.[28] We observed diffuse endocapillary proliferative GN in two cases causing AKI.
In summary, AKI in an HIV patient may relate to hypovolemia, sepsis, drug toxicity, ischemic/toxic ATN, tubulointerstitial injury and GN. We observed incidence of AKI in HIV-positive patient was 3.9% in the present study. Majority of AKI was related to prerenal factors leading to ischemic injury (ATN) in the kidney. Acute GN and AIN were less common causes of AKI, while TMA was a very uncommon disease causing AKI in HIV patients. In-hospital mortality was 24.64% and mostly related to AIDS-associated opportunistic infections. Prognosis of AKI was favorable with full recovery of renal functions in 59% of cases. Twenty (14.5%) patients developed Stage 3-5 CKD and 3 (2.2%) cases progressed to ESRD, 3 months after episode of AKI.
Source of Support: Nil
Conflict of Interest: None declared.
References
- A history of AIDS: Looking back to see ahead. Eur J Immunol. 2007;37(Suppl 1):S94-102.
- [Google Scholar]
- Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27-34.
- [Google Scholar]
- Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99-106.
- [Google Scholar]
- Acute kidney injury in hospitalized HIV-infected patients: A cohort analysis. Nephrol Dial Transplant. 2011;26:3888-94.
- [Google Scholar]
- Kidney disease in the HIV-infected patient. AIDS Patient Care STDS. 2007;21:813-24.
- [Google Scholar]
- Renal disease in patients with HIV infection: Epidemiology, pathogenesis and management. Drugs. 2008;68:963-80.
- [Google Scholar]
- Immunodeficiency and renal impairment are risk factors for HIV-associated acute renal failure. AIDS. 2010;24:2239-44.
- [Google Scholar]
- Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005;67:1526-31.
- [Google Scholar]
- Acute kidney injury. In: Tall MW, Chertow GM, Marsden PA, Skoreck K, Yu Asi, Brenner BM, eds. Brenner and Rector's the Kidney (9th ed). Philadelpia: Saunders; 2012. p. :1064-5.
- [Google Scholar]
- Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292-8.
- [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
- [Google Scholar]
- Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-55.
- [Google Scholar]
- 2007. Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. Available from: http://www.nacoonline.org/Quick_Links/Publication/Treatment_Care__Support/Operational_Technical_guidelines_and_policies/Antiretroviral_Therapy_Guidelines_for_HIV_infected
- 2013. Global report UNAIDS report on global AIDS epidemic, 2013. Available from: http://www.unaids.org/en/dataanalysis/knowyourepidemic/epidemiologypublications/
- 2012. HIV and AIDS estimates 2013. Available from: http://www.unaids.org/en/regionscountries/countries/india/
- Acute renal failure in hospitalized patients with HIV: Risk factors and impact on in-hospital mortality. AIDS. 2006;20:561-5.
- [Google Scholar]
- Acute renal failure due to indinavir crystalluria and nephrolithiasis: Report of two cases. Am J Kidney Dis. 1997;30:558-60.
- [Google Scholar]
- Fanconi syndrome and renal failure induced by tenofovir: A first case report. Am J Kidney Dis. 2002;40:1331-3.
- [Google Scholar]
- Drug rash with eosinophilia and systemic symptoms syndrome and renal toxicity with a nevirapine-containing regimen in a pregnant patient with human immunodeficiency virus. Obstet Gynecol. 2003;101:1094-7.
- [Google Scholar]
- Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: A cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831-7.
- [Google Scholar]
- Renal failure in HIV-positive patients-A South African experience. Clin Kidney J. 2013;6:584-9.
- [Google Scholar]
- Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171-7.
- [Google Scholar]
- Acute renal failure in the course of HIV infection: A single-institution retrospective study of ninety-two patients and sixty renal biopsies. Nephrol Dial Transplant. 1999;14:1578-85.
- [Google Scholar]







