Translate this page into:
Acute Lymphatic Leukemia Masquerading as Recurrent Acute Kidney Injury – A Case Report
Corresponding author: Ranjit Narayanan, Department of Nephrology, Iqraa International Hospital and Research Centre, Malaparamba PO, Calicut, Kerala, India. E-mail: ranjitnarayanan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Narayanan R, Hafeeq B, Gopinathan JC, Aziz F, Krishnakumar A, Aboobacker IN, et al. Acute Lymphatic Leukemia Masquerading as Recurrent Acute Kidney Injury – A Case Report. Indian J Nephrol. doi: 10.25259/IJN_237_2024
Abstract
Acute kidney injury (AKI) is an uncommon presenting feature in acute lymphatic leukemia (ALL). We report an unusual case of a 15-year-old girl who has experienced multiple episodes of AKI over an 8 month period with unremarkable WBC counts. She now returned with constitutional symptoms and rapidly progressive renal failure with bulky kidneys, proteinuria, and sterile pyuria. A renal biopsy revealed diffuse interstitial infiltration with CD3 positive, TdT positive, and CD20 negative lymphoblasts, suggestive of acute T cell leukemia. Bone marrow studies and flow cytometry later confirmed the diagnosis of T cell ALL. Unfortunately, she succumbed to an intracranial bleed during the intensive phase of chemotherapy. In addition to the atypical presentation of ALL, this case highlights the importance of timely renal biopsy in cases of AKI, where the cause is unclear.
Keywords
Acute kidney injury
Recurrent AKI
Acute lymphatic leukemia
Renal biopsy
Introduction
Acute kidney injury (AKI) as the initial presentation of acute lymphocytic leukemia (ALL) is uncommon, though reported earlier.1–4 We describe an unusual presentation of T-cell ALL presenting with recurrent episodes of AKI.
Case Report
A 15-year-old girl visited our hospital in June 2021 with fever and fatigue for 3 days. She was pale with cushingoid facies, mild pedal edema, and stable vital parameters. A 2x2 cm left axillary lymph node was palpable. Her systemic examination was otherwise unremarkable.
Her past medical history was remarkable, having been hospitalized thrice elsewhere in the previous 8 months with fever and AKI [Table 1].
| First hospitalization | Follow-up | Second hospitalization | Follow-up | Third hospitalization | Follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18/11/20 | 21/11/20 | 23/11/20 | 30/11/20 | 3/2/21 | 10/2/21 | 14/2/21 | 26/2/21 | 30/4/21 | 1/5/21 | 8/5/21 | 14/5/21 | 25/5/21 | |
| Hb (mg/dL) | 8.1 | 8.4 | 6.7 | 7.4 | 7.5 | 8.6 | 9.6 | 9.2 | |||||
| WBC (/mm3) | 5500 | 6800 | 2980 | 12410 | |||||||||
| DLC | P70L24M5 | P62L28M5 | P80L13M1 | P66L22M10 | |||||||||
| Platelets (*103/mm3) | 285 | 373 | |||||||||||
| Urea (mg/dL) | 93 | 210 | 104 | 65 | 28 | 165 | 192 | 156 | 73 | 31 | |||
| Creat (mg/dL) | 5.8 | 3.1 | 1.9 | 0.9 | 8.3 | 3.4 | 1.7 | 0.76 | 7.8 | 10.8 | 4.2 | 2.4 | 1.6 |
| Na (meq/L) | 140 | 141 | 121 | 129 | 133 | 141 | 136 | 140 | 129 | 139 | 139 | ||
| K (meq/L) | 6.2 | 4.5 | 3.9 | 3.5 | 4.9 | 2.6 | 3.0 | 3.3 | 4.2 | 4.7 | 2.1 | 3.3 | 4.1 |
| Ca (mg/dL) | 8.9 | 9.3 | 7.0 | 9.7 | |||||||||
| P (mg/dL) | 5.9 | 2.3 | 13.5 | ||||||||||
| Bilirubin (mg/dL) | 1.9/1.7 | 0.34 | |||||||||||
| AST/ALT (IU/L) | 134/176 | 10/12 | |||||||||||
| ALP (IU/L) | 1839 | 129 | |||||||||||
| TP/A/G (g/dL) | 7/3.5/3.5 | 7.5/3.5 | |||||||||||
Hb: Hemoglobin, WBC: White blood cell count, DLC: Differential leucocyte count, P: Polymorphs, L: Lymphocytes, M: Monocytes, Creat: creatinine, Na: sodium, K: potassium, Ca: calcium, P: phosphorus, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, ALP: Alkaline phosphatase, TP: Total protein, A: albumin, G: globulin.
She initially presented in November 2020 with fever, vomiting, anemia, azotemia, and bland urine. Ultrasound showed bulky kidneys without obstruction. Peripheral smear showed dimorphic anemia. Antinuclear antibody (ANA) was negative and C3 complement was normal. AKI resolved spontaneously. Serum creatinine improved to 0.9 mg/dL.
In February 2021, she developed fever, fatigue, vomiting, and breathlessness. Evaluation revealed advanced renal failure, transaminitis, severe anemia, and leukopenia. Hematologic workup was not done. She recovered with dialytic support, blood transfusions, empiric steroids, and antibiotics. Her cultures were negative. Serum creatinine improved to 0.76 mg/dL.
She presented next in May 2021 with fever, vomiting, altered sensorium, severe renal failure, and anemia. She improved again with temporary dialytic support and steroid dose enhancement. Serum creatinine declined to 1.6 mg/dl.
On her current admission, she had leucocytosis, azotemia [Table 2], albuminuria and pyuria. Serum calcium and uric acid levels were normal. Serum complement levels, ANA, ANA profile, and ANCA serology were unremarkable. Ultrasound showed bulky kidneys with increased echogenicity. Blood and urine cultures were sterile.
| 5/6/21 | 7/6/21 | 13/6/21 | 14/6/21 | 15/6/21 | 16/6/21 | 19/6/21 | |
|---|---|---|---|---|---|---|---|
| Hb (mg/dL) | 10.1 | 9.4 | 8.8 | 8.6 | 8.2 | 8.1 | 7.9 |
| WBC (/mm3) | 21800 | 11700 | 31200 | 33600 | 33100 | 36000 | 28400 |
| DLC | P58L30M12 | P85L13M2 | P69/L21/M10 | P64L22M14 | P77L17M6 | P75L20M5 | P63L30M7 |
| Platelets (*103/mm3) | 151 | 170 | 179 | 178 | 163 | 150 | |
| ESR (1st hour) | 100 | ||||||
| Urea (mg/dL) | 158 | ||||||
| Creat(mg/dL) | 5.59 | 6.52 | 5.44 | 4.93 | 4.28 | 4.52 | 5.78 |
| Na (meq/L) | 140 | 139 | |||||
| K (meq/L) | 4.55 | 5.18 | 3.46 | 3.39 | 3.56 | 3.69 | 3.29 |
| Ca (mg/dL) | 8.3 | 9.2 | 9.2 | 8.7 | |||
| P (mg/dL) | 2.5 | 1.5 | 1.0 | 1.5 | |||
| Uric acid (mg/dL) | 4.3 | 45.8 | 18 | 24.9 | 12.2 | 9.3 | |
| Mg (meq/L) | 1.74 | ||||||
| Bilirubin (mg/dL) | 0.18 | ||||||
| AST/ALT (IU/L) | 18/112 | ||||||
| ALP (IU/L) | 213 | ||||||
| TP/A/G (g/dL) | 6.2/3.4/2.8 | ||||||
| LDH (IU/L) | 1314 | ||||||
| Urine examination | |||||||
| Albumin | ++ | ||||||
| Pus Cells/HPF | numerous | ||||||
| RBC/HPF | 0-1 | ||||||
| PC ratio | 3.98 |
Hb: Hemoglobin, WBC: White blood cell count, DLC: Differential leucocyte count, P: Polymorphs, L: Lymphocytes, M: Monocytes, ESR: Erythrocyte sedimentation rate, Creat: creatinine, Na: sodium, K: potassium, Ca: calcium, P: phosphorus, Mg: magnesium, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, ALP: Alkaline phosphatase, TP: Total protein, A: albumin, G: globulin, LDH: Lactate dehydrogenase, RBC: Red blood cells, HPF-High power field, PC ratio: Protein creatinine ratio.
She underwent hemodialysis on Days 2 and 4 and a renal biopsy was performed on Day 5. She was given empiric pulse methylprednisolone (125 mg) on day 6 and 7 for rapidly progressive renal failure. On day 7, the renal biopsy was provisionally reported as diffuse interstitial infiltration with atypical lymphoid cells, suggestive of leukemia.
On day 8, WBC counts increased to 33,100/mm3 and uric acid to 45 mg/dL. Further doses of methylprednisolone were withheld. She remained normocalcemic with hypophosphatemia of 2.5 mg/dL which later reduced to 1 mg/dL. The LDH level was elevated at 1314 U/L. Peripheral smear showed 11% blasts and a bone marrow study was performed. Hyperuricemia was managed with parenteral Rasburicase and prolonged dialysis sessions. Thereafter, she remained off dialysis though stable azotemia persisted.
Her renal biopsy [Figure 1] finally showed diffuse interstitial infiltration by atypical monomorphic lymphoid cells positive for CD3 and terminal deoxynucleotidyl transferase (TdT) and negative for CD20 by immunohistochemistry, suggestive of T-cell-ALL. Peripheral smear, bone marrow examination, and flow cytometry reports were consistent with acute T-cell ALL [Figure 2].
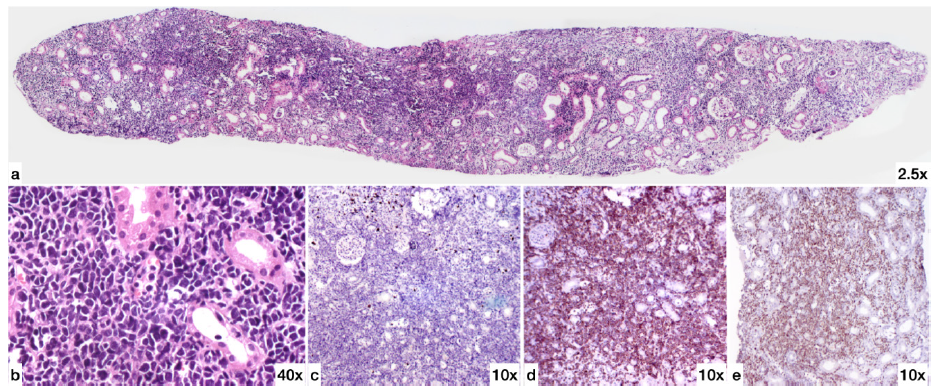
- Renal Biopsy (a) H&E (2.5X magnification): One core of renal tissue showing interstitium diffusely infiltrated by atypical small round blue cells. (b) H&E High power (40X magnification) showing the atypical cells to be monomorphic lymphoid cells with scant amount of cytoplasm and irregular large hyperchromatic nucleus. The cells are 2.5 to 3 times size of small mature lymphocytes and have irregular nuclear contours. (c) CD20 (10X magnification) The atypical lymphoid cells are negative for CD20. (d) CD3 (10X magnification) The atypical lymphoid cells are strongly positive for CD3. (e) TdT (10X magnification) The atypical lymphoid cells are positive for terminal deoxynucleotidyl transferase (TdT). H&E: Hematoxylin & Eosin.
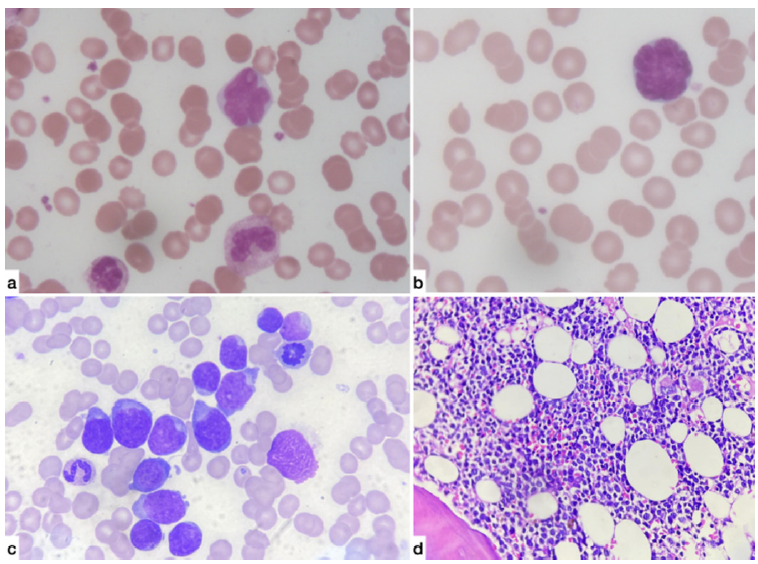
- (a & b) Peripheral smear (100X magnification): Atypical cells having 2.5 to three times the size of small mature lymphocytes with scant to moderate cytoplasm, highly irregular nuclear membranes and opened out chromatin with many showing prominent nucleoli. (c) Bone marrow Aspirate (100X magnification): Blasts with scant to moderate bluish cytoplasm and irregular opened out nucleus with 1-2 prominent nucleoli. (d) Bone marrow Trephine (40X magnification): Shows sheets of atypical monomorphic lymphoid cells irregular nuclear contours and prominent nucleoli.
She was transferred to pediatric oncology services for further care. She succumbed to an intracranial bleed during the induction phase of ALL treatment.
Discussion
Leukemic infiltration of the kidney is usually subclinical and often an autopsy finding.1 AKI due to leukemic infiltration in ALL is uncommon (<1%),1 though previously reported.2,3,4
Our case is unique for its presentation with recurrent episodes of AKI over an extended period. The absence of overt leukemic manifestations, spontaneous resolution of AKI on her first presentation, and response to steroids on subsequent occasions possibly hindered an earlier diagnosis. Initial presentation of ALL with AKI and normal WBC counts, although uncommon, has been reported.5,6 Arguably, abnormal cells may have been missed by automated counters but could have been detected by an earlier peripheral smear.
During this admission, at the outset, there was no evidence of usual causes of AKI in ALL like tumor lysis, hyperuricemia, volume depletion, sepsis, or obstruction. Biopsy findings confirmed that her AKI was entirely due to leukemic infiltration of the kidneys. Her kidneys were consistently bulky on imaging, strongly suggesting that the previous AKI episodes were also attributable to leukemic kidney infiltration. An earlier kidney biopsy would have expedited the diagnosis.
Administering pulse steroids unravelled very high WBC counts and severe hyperuricemia with high LDH levels, indicating tumor lysis syndrome. Concurrent hypophosphatemia was likely due to the incorporation of phosphorus into rapidly growing tumor cells.2,7 Therefore, caution is warranted when prescribing empiric pulse steroids when the cause of AKI is uncertain.
AKI at initial presentation usually indicates a worse prognosis for ALL.2,8 Our patient succumbed during the induction phase of ALL treatment.
Conclusion
Our case highlights an unusual presentation of ALL with recurrent episodes of AKI. A kidney biopsy was crucial in confirming the diagnosis, especially in the absence of clear indicators of a hematological malignancy. This emphasizes the importance of timely kidney biopsy when the etiology of AKI is unclear.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis.. 2014;21:27-35.
- [CrossRef] [PubMed] [Google Scholar]
- Acute lymphoblastic leukemia presenting as acute renal failure. Nat Clin Pract Nephrol.. 2007;3:106-10.
- [CrossRef] [PubMed] [Google Scholar]
- Acute renal failure due to leukemic cell infiltration followed by relapse at multiple extramedullary sites in a child with acute lymphoblastic leukemia. Leuk Lymphoma.. 2004;45:825-8.
- [CrossRef] [PubMed] [Google Scholar]
- Leukemic infiltration of kidney in a case of T-cell acute lymphomatous leukemia. J Postgrad Med Edu Res.. 2020;54:59-61.
- [Google Scholar]
- Acute renal failure associated with bilateral enlargement of the kidneys: A rare manifestation of acute lymphoblastic leukemia (ALL) Klin Padiatr.. 2009;221:176-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute renal failure and normal blood count: A rare presentation of T-cell acute lymphoblastic leukemia. Leuk Res Rep.. 2013;3:14-16.
- [CrossRef] [PubMed] [Google Scholar]
- Alterations in electrolyte equilibrium in patients with acute leukemia. Eur J Haematol.. 2005;75:449-60.
- [CrossRef] [PubMed] [Google Scholar]
- Acute kidney injury in patients with newly diagnosed high-grade hematological malignancies: Impact on remission and survival. PLoS ONE.. 2013;8:e55870.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







