Translate this page into:
An Uncommon Complication of a Common Tropical Infection in a Kidney Transplant Recipient – A Case Report
Corresponding Author: Dr. Mythri Shankar, Department of Nephrology, Institute of Nephrourology, Bengaluru, Karnataka, India. E-mail: mythri.nish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shankar M, Gurusiddiah SC, Nayaka M, Aralapuram K. An Uncommon Complication of a Common Tropical Infection in a Kidney Transplant Recipient – A Case Report. Indian J Nephrol 2024;34:79-83. doi: 10.4103/ijn.ijn_252_22
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening condition due to extensive and uncontrolled immune activation. There is sparse literature on HLH in kidney transplant recipients. We report a case of a 27-year -old male kidney transplant recipient who presented with dengue fever and acute allograft dysfunction. Following improvement in allograft function with supportive treatment, he was found to have worsening pancytopenia with unusually high serum ferritin levels. Bone marrow aspiration performed for pancytopenia revealed hemophagocytosis. A diagnosis of HLH secondary to dengue viral infection was made based on the modified HLH diagnostic criteria (2009). He received supportive treatment and steroids and was discharged in a stable condition with normal kidney allograft functions. To our knowledge, this is the first case report of HLH secondary to dengue viral infection in a kidney allograft recipient managed successfully with timely diagnosis and appropriate treatment.
Keywords
Dengue
hemophagocytosis
transplant
Introduction
Hemophagocytic syndrome, also called hemophagocytic lymphohistiocytosis (HLH), is a rare, life-threatening condition caused due to an uncontrolled and aggressive immune response.1 Its pathogenesis is due to the impaired function of cytotoxic T lymphocytes and natural killer (NK) cells. Despite the activation of the immune system, it is unable to remove the inciting antigens. Also, there is impaired modulation and termination of the immune system. This causes an increased production of proinflammatory cytokines and proliferation of histiocytes.1,2
HLH is classified as primary and secondary. Primary HLH is caused by genetic mutations that affect the function of cytotoxic T lymphocytes and NK cells and is typically seen in children. Secondary HLH (sHLH) may occur at any age due to conditions that cause strong immune system activation or an immunodeficiency state.1,2 The most common triggering conditions for sHLH are malignancies, followed by infections and autoimmune diseases.3 Rarely, it can present in patients with strong immunosuppression, such as organ transplant recipients. Hyperinflammation and aggressive histiocytosis lead to hematological disturbance and massive tissue damage. It is a life-threatening condition, and prompt treatment is critical. A significant barrier is a delay in diagnosis due to nonspecific symptoms and signs, posing a challenge even for experienced clinicians.3
There is scarce literature on sHLH in kidney transplant recipients.4-7 The most prominent case series involves 17 patients of sHLH in a cohort of 4230 kidney transplant recipients.8 Among the 17 patients, nine had sHLH due to viral infection (cytomegalovirus [CMV], Epstein– Barr virus [EBV], human herpes virus 6, and human herpes virus 8), three had sHLH due to bacterial infection (tuberculosis and Bartonella henselae), two patients had other infections (toxoplasmosis and Pneumocystis carinii pneumonia), and posttransplant lymphoproliferative disease was seen in two other patients. Death occurred in 47% of the cases despite treatment of infections and reduction of immunosuppression.8 Unlike all the cases reported to date, in this case, sHLH was triggered by a dengue viral infection. To the best of our knowledge, this is the first case report of sHLH in a kidney transplant recipient due to dengue infection. This patient was successfully treated with steroids, and he completely recovered.
Case Report
A 27-year-old male deceased donor kidney transplant recipient presented to us 6 months posttransplant with high-grade fever, retro-orbital pain, headache, and myalgia of 3 days duration. His brother, who lives with him, had similar complaints, and was diagnosed with dengue fever. His native kidney disease was IgA nephropathy. His dialysis vintage was 6 years. He received basiliximab induction and was on triple-drug immunosuppression (mycophenolate mofetil, tacrolimus, steroids). He had immediate graft function with nadir creatinine of 0.8 mg/dl. There was no history of abdominal pain, dysuria, or yellowish discoloration of urine or sclera. Also, there was no history of arthralgia, rash, and seizures/altered sensorium. On general physical examination, he showed signs of dehydration, hypotension with a blood pressure of 90/60 mmHg, and tachycardia. Systemic examination was unremarkable. Routine laboratory tests revealed pancytopenia, acute allograft dysfunction, hyponatremia, and sinus tachycardia on electrocardiogram (ECG) [Table 1]. Tacrolimus trough level was 7 ng/ml. Ultrasound abdomen and pelvis was suggestive of mild splenomegaly. Intravenous fluid therapy was started immediately, which consequently led to improvement in blood pressure. Tacrolimus dose was reduced, mycophenolate mofetil was stopped, and a stress dose of steroids was initiated. Because of thrombocytopenia, investigations were sent to look for tropical infections. Dengue NS1 antigen returned positive. Workup for leptospirosis, malaria, and scrub typhus was negative. Blood and urine cultures were sterile. Hence, a diagnosis of dengue fever was made. During the ward course, kidney functions and blood pressure normalized. However, fever persisted, pancytopenia continued to worsen, and inflammatory markers such as hsCRP and D-dimer were rising [Table 2]. He developed mucosal bleed with petechiae due to thrombocytopenia, which required transfusion of single donor platelets. Among all the blood tests performed, a few were particularly noteworthy – serum ferritin level 26,250 ng/ml and lactate dehydrogenase (LDH) level 665 U/l. Because of recovered dengue fever and allograft dysfunction, but worsening pancytopenia, he underwent a bone marrow biopsy. Simultaneously, we looked for CMV, EBV, and parvovirus DNA polymerase chain reaction (PCR) levels, which returned negative.
| Day 1 | |||
|---|---|---|---|
| Hemoglobin (g%) | 10.4 | S. Corrected calcium (mg/dl) | 8.4 |
| Total WBC count (cells/mm3) | 2000 | S. Phosphorous (mg/dl) | 3 |
| Differential count | 66% polymorphs | S. Uric acid (mg/dl) | 5.5 |
| Platelet count (cells/mm3) | 39000 | Urine routine | Protein: nil |
| Pus cells 0-1 | |||
| RBCs : nil | |||
| Hematocrit | 30.4% | HIV | Negative |
| ESR (mm/hr) | 55 | HbsAg | Negative |
| Urea (mg/dl) | 106 | HCV | Negative |
| Creatinine (mg/dl) | 2.1 | Procalcitonin (ng/ml) | 1.99 |
| Sodium (mEq/L) | 127 | Peripheral smear | Normocytic normochromic anemia |
| Leukocytes :reduced in number, no atypical cells | |||
| Platelets reduced in number | |||
| Potassium (mEq/L) | 3.8 | PT, Aptt, INR | Within normal limits |
| Total bilirubin (mg/dl) | 1.0 | Vitamin B12 levels (pg/ml) | 900 |
| Direct bilirubin (mg/dL) | 0.5 | COVID RT PCR | Negative |
| SGOT (IU/L) | 49 | Chest X ray | Within normal limits |
| SGPT (IU/L) | 35 | ECG | Sinus tachycardia |
| Total protein (g/dl) | 6.3 | ||
| S. Albumin (g/dl) | 3.5 | ||
| Reticulocyte count % | 0.9 | ||
| Magnesium (mg/dl) | 1.68 | ||
| Folate levels (ng/ml) | 6.7 (normal) | ||
WBC: White blood cells; HCV: Hepatitis C virus; SGOT: Serum glutamate, oxaloacetic transaminase; SGPT: Serum pyruvate transminase; ECG: Electrocardiogram, PT: Prothrombin time, INR: International normalised ratio, Aptt: Activated partial thromboplastin time
| Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|
| Blood urea (mg/dl) | 106 | 38 | 36 | 57.7 |
| Serum creatinine (mg/dl) | 2.1 | 1.56 | 1.05 | 0.79 |
| Serum sodium (mEq/L) | 127 | 129 | 132 | 132 |
| Serum potassium (mEq/l) | 3.8 | 3.8 | 4.0 | 4.0 |
| Total bilirubin/Direct bilirubin (mg/dl) | 1.1/0.5 | 1.18/0.9 | 1.2/1.7 | 1.2/0.7 |
| SGOT/SGPT (IU/L) | 49/35 | 58/27 | 50/27 | 40/23 |
| Total protein/serum albumin (g/dl) | 6.3/3.6 | 5.1/3.6 | 5.5/3.4 | 5.6/3.2 |
| Hemoglobin (g/dl) | 11.4 | 12.6 | 11.2 | 10.7 |
| Total count (cells/mm3) | 2000 | 1680 | 1300 | 700 |
| Differential count (% of polymorphonuclear cells) | 66 | 63 | 77 | 41 |
| Platelet count (cells/mm3) | 39000 | 6000(SDP transfused) | 8000 | 27000 |
| Hematocrit % | 30.4 | 30.2 | ||
| S.Ferritin (ng/ml) | 26250 | |||
| hsCRP (normal <1mg/dl) | 3.54 | |||
| D-dimer (normal <0.4 μg/L) | 0.24 | |||
| S.Triglyceride levels (mg/dl) | 280 |
SGOT: Serum glutamate, oxaloacetic transaminase; SGPT: Serum pyruvate transminase
Bone marrow aspiration was suggestive of hypocellular marrow with erythroid predominance and maturation arrest in the granulocyte series with evidence of hemophagocytosis [Figure 1]. The peripheral smear did not show any features of hemophagocytosis. A diagnosis of HLH secondary to dengue viral infection was made based on the modified HLH 2009 criteria [Table 3]. This patient had fever, splenomegaly, pancytopenia, elevated ferritin levels, hypertriglyceridemia, and hemophagocytosis on bone marrow aspiration, satisfying the diagnostic criteria for HLH.
| MODIFIED 2009 HEMOPHAGO LYMPHO HISTIOCYTOSIS (HLH) CRITERIA |
|---|
| At least three of the following : |
| Fever |
| Splenomegaly |
| Cytopenia affecting at least two cell lines : |
| Hemoglobin <9g/l |
| Platelets <100 b /l |
| Absolute neutrophil count <1000 b/l |
| Hepatitis |
| At least one of the following : |
| Ferritin elevation - |
| Elevated soluble CD25 (Soluble IL2 –receptor) |
| Hemophagocytosis seen on tissue biopsy |
| Low /absent NK -cell activity |
| Other supportive features (not required) |
| Hypertriglyceridemia |
| Hypofibrinogenimia |
| Hyponatremia |
HLH = hemophagocytic lymphohistiocytosis
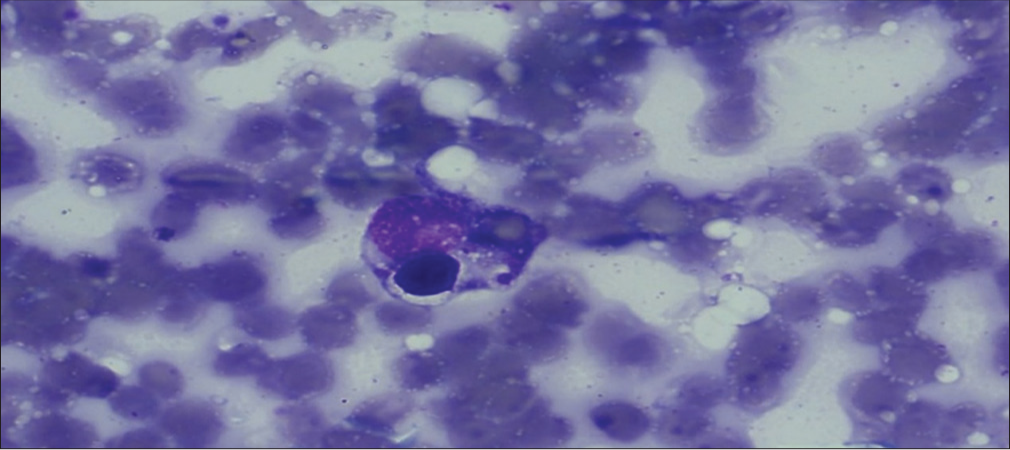
- Hemophagocytosis on bone marrow aspiration.
He was started on intravenous (IV) dexamethasone 10 mg/m2 (as per the HLH 2004 protocol), which was tapered over 10 days. Following this, the fever subsided, and the patient improved symptomatically. Pancytopenia improved over the next 3 days. HLH markers repeated every 3 days showed an improving trend. He was discharged in a stable condition with normal allograft function and reduced levels of inflammatory markers on day 10. On follow-up visits, the tacrolimus dose was optimized and mycophenolate mofetil was restarted approximately 1 week after discharge.
Discussion
Dengue virus infection is a global threat caused by a virus from the flaviviridae family called the arbovirus transmitted by Aedes aegypti and Aedes albopictus mosquitoes in the endemic South Asian countries.9
Dengue infection in transplant patients may pose various challenges. Dengue viremia may be more prolonged than commonly observed in non-transplant recipients. The severity of infection may be more in transplant recipients, particularly in the early posttransplant period. They are more prone to dengue hemorrhagic fever and shock syndrome. In addition to supportive care with fluid therapy, transplant recipients require reduced immunosuppression and strict monitoring for the development of capillary leak syndrome.9,10
Cornelia et al.11 showed that dengue viral infection could cause hyperferritinemia. Increased ferritin levels were associated with highly active disease-causing immune activation and coagulation disturbances. sHLH can be triggered by a viral infection such as dengue and is also associated with hyperferritinemia. The diagnosis may be difficult in such situations.
sHLH is rarely seen in patients on immunosuppression therapy.4,12 The most common causes reported among kidney transplant recipients are EBV and CMV infection. In this case, it was a dengue viral infection.
Also, in this case, the diagnosis was delayed due to dengue infection, which had triggered HLH. This patient presented with pancytopenia and acute allograft dysfunction. The most common causes for pancytopenia and acute allograft are immunosuppressants, CMV infection, and anti-CMV drugs, which were ruled out in this patient. The only suspicion was that the lab value of ferritin was unusually high (26250 ng/ml). The final step was bone marrow aspiration to establish the diagnosis of HLH. Hemophagocytosis is the hallmark of HLH. HLH in adults is diagnosed based on the modified HLH 2009 criteria [Table 3].
The goal of treatment is to reduce inflammation and destroy the immune cells causing life-threatening inflammation. In deteriorating and acutely ill patients, HLH-94 protocol-based treatment is preferred; dexamethasone and etoposide are given weekly until improvement and gradually weaned off. In case of nonrecovery, the therapy is continued as a bridge for hematopoietic stem cell transplant.13 If the patient is not critical, initiate steroids and monitor for treatment response, rather than starting chemotherapy. Treatment of the triggering condition improves the clinical condition in some cases.14 In this case, since the patient was stable, he was initiated on steroid therapy and showed signs of improvement in 3 days. Supportive care in the form of transfusions for anemia and thrombocytopenia should be given when required.14 This patient received platelet transfusions, due to thrombocytopenia and bleeding manifestations.
Conclusion
Secondary HLH is a poorly understood, life-threatening, and rare condition. Further research is required in its diagnosis and treatment. Its clinical course can be variable in kidney allograft recipients. It is essential to consider sHLH in transplant recipients presenting with nonspecific symptoms, cytopenia, and marked elevation of inflammatory markers (sepsis-like clinical picture). Once the diagnosis is established, look for the triggering condition. Prompt treatment of the triggering factor is equally essential, and response to therapy should be monitored.
Acknowledgment
We acknowledge the Director, all faculty and residents of Institute of Nephro-urology, Bengaluru for their administrative and technical help in the workup of this case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Approach to hemophagocytic syndromes. Hematology Am Soc Hematol Educ Program. 2011;2011:178-83.
- [CrossRef] [PubMed] [Google Scholar]
- Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: A case report. Medicine. 2017;96:e9198.
- [CrossRef] [PubMed] [Google Scholar]
- How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908-14.
- [CrossRef] [PubMed] [Google Scholar]
- Secondary hemophagocytic syndrome after renal transplantation: Two case-reports. J Bras Nefrol. 2020;42:118-23.
- [CrossRef] [PubMed] [Google Scholar]
- Haemophagocytic syndrome-A life-threatening complication of renal transplantation. Nephrol Dial Transplant. 2009;24:2623-7.
- [CrossRef] [PubMed] [Google Scholar]
- Hemophagocytic lymphohistiocytosis in an adult kidney transplant recipient successfully treated by plasmapheresis: A case report and review of the literature. Medicine (Baltimore). 2017;96:e9283.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual presentation of hemophagocytic lymphohistiocytosis in a kidney transplant patient. Case Rep Transplant. 2019;2019:3682378.
- [CrossRef] [PubMed] [Google Scholar]
- Hemophagocytic syndrome in renal transplant recipients: Report of 17 cases and review of literature. Transplantation. 2004;77:238-43.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue infection immediately after kidney transplantation. Transplantation. 2022;106:e354-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dengue in Renal Allograft Transplant Recipients and the Effect of Dengue Screening in Both Donor and Recipient and Its Outcome after Transplant [abstract] ISOT : 2018 [Internet]. IJT Online: 2018. New Delhi. :256. Available from: https://www.ijtonline.in [Last accessed on 2022 Oct 13]
- [Google Scholar]
- Hyperferritinaemia in dengue virus infected patients is associated with immune activation and coagulation disturbances. PLoS Negl Trop Dis. 2014;8:e3214.
- [CrossRef] [PubMed] [Google Scholar]
- Mycophenolate mofetil: A possible cause of hemophagocytic syndrome following renal transplantation? Am J Transplant. 2010;10:2378-9.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100:2367-73.
- [CrossRef] [PubMed] [Google Scholar]
- How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041-52.
- [CrossRef] [PubMed] [Google Scholar]







