Translate this page into:
Anti-C1q antibodies and their association with complement components in Indian systemic lupus erythematosus patients
Address for correspondence: Dr. Vandana Pradhan, Department of Autoimmune Disorders, National Institute of Immunohematology, Indian Council of Medical Research, Floor #13, KEM Hospital, Parel, Mumbai- 400 012, Maharashtra, India. E-mail: pradhanv69@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Systemic lupus erythematosus (SLE) is a prototype autoimmune disease, characterized by immune complex formation and systemic inflammation. Complement components such as C1q and mannose-binding lectin (MBL) play an important role in the clearance of immune complexes. Anti-C1q antibodies are associated with lupus nephritis and reduced levels of the complement components. The objective of this study was to detect anti-C1q antibodies in SLE patients and to evaluate their association with the complement components. Sixty SLE patients were included, of whom 75% had lupus nephritis (LN) and 25% were without renal manifestations (non-LN). The disease activity was assessed at the time of evaluation by the systemic lupus erythematosus disease activity index (SLEDAI). Anti-C1q antibodies, circulating immune complexes, and serum MBL levels were detected by enzyme-linked immunosorbent assay. The anti-C1q antibody prevalence was 58.3%. The LN patients showed 60% anti-C1q positivity with a higher percentage in membranoproliferative glomerulonephritis patients (51.9%). Anti-dsDNA positivity was slightly higher among the anti-C1q positives than in the anti-C1q negatives (65.7% vs. 60%). A higher percentage of reduced C3 and C4 levels was noted among the anti-C1q positives. The LN patients showed a higher percentage of low MBL levels among anti-C1q negatives than in the anti-C1q positives (61.1% vs. 55.6%). Non-LN patients showed a higher percentage of low MBL levels among anti-C1q positives than among anti-C1q negatives (87.5% vs. 57.1%). Anti-C1q antibodies were found in both LN and non-LN patients, but there was no correlation with the clinical severity of the disease.
Keywords
Anti-C1q antibodies
C3
C4
lupus nephritis
mannose-binding lectin
SLE without nephritis (non-LN)
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a prototype autoimmune disease characterized by the increased production of multiple autoantibodies mainly directed against nuclear antigens. This initiates the formation and deposition of circulating immune complexes (CICs) that further leads to an intense inflammatory response and tissue damage.[12] Complement activation in SLE is predominantly due to the interaction of a C1q component with the immune complexes, which is the first component of a classical complement pathway. Deficiency of C1q leads to autoimmunity, associated with impaired apoptotic clearance and appearance of glomerular apoptotic bodies. Deficiency of classical complement components such as C1q and C4 is strongly associated with the pathogenesis of SLE.[3–6] Mannose-binding lectin (MBL), an acute phase protein, is responsible for complement activation via the lectin pathway. The MBL plays an important role in the clearance of immune complexes. The MBL is also reported to facilitate apoptotic cell clearance. In recent times, MBL deficiency has emerged as a probable cause for SLE susceptibility.[17]
Anti-C1q antibodies are directed against the collagen-like region of C1q and are strongly correlated with hypocomplementemia and renal flares, suggesting that they may play a pathogenic role. Anti-C1q antibodies do not seem to activate the complement, but their binding to C1q may amplify complement activation as well as attenuate the physiological functions of C1q. Earlier studies have suggested that due to the prevalence of anti-C1q antibodies in SLE, especially in patients with renal involvement, they may play a possible role as biomarkers in lupus nephritis (LN).[89] However, some reports state that anti-C1q antibodies are required but not sufficient for the development of renal flares and that anti-dsDNA autoantibodies, along with elevated levels of anti-C1q antibodies, are associated with renal disease.[10–12] This study was conducted to determine the prevalence of anti-C1q antibodies in Indian SLE patients and their association with complement components such as C3, C4, and MBL.
Materials and Methods
Patients and controls
This study was conducted on 60 patients diagnosed with SLE, who were referred to our center in Mumbai, India, for a period of three years (2008-2010). All patients showed elevated levels of CICs. The SLE patients were diagnosed according to the American College of Rheumatology (ACR) criteria.[13] The study was carried out after obtaining the requisite Ethics Committee approval and a written consent from the patients. Disease activity was assessed at the time of evaluation, by using the systemic lupus erythematosus disease activity index (SLEDAI).[14] The disease activity in all SLE patients was classified as mild, moderate, or severe, based on their SLEDAI scores (mild <8, moderate 8-18, and severe >18). Pregnant and postmenopausal women, smokers, patients with diabetes, and patients with significant hyperlipidemia were excluded. After blood collection, the sera were stored in aliquots at -80°C, until they were tested. Renal biopsies of the LN cases were examined by light microscopy using hematoxylin and eosin, and periodic schiff (PAS) staining. Immunofluorescence microscopy was performed using anti-IgG, anti-IgM, anti-IgA, anti-C3, anti-C4, and anti-fibrinogen fluorescein isothiocyanate conjugate (FITC). In LN patients, renal histology was classified according to WHO criteria.[15] Normal control sera were obtained from 50 healthy individuals from the blood bank.
Detection of CICs was carried out using IgG-CIC enzyme-linked immunosorbent assay (ELISA)(DiaMetra, Italy). Anti-C1q antibodies were measured using Autostat II C1q-CIC, Hycor Biomedical Inc., California, USA. All the samples were tested for serum; the MBL levels were measured using the MBL Oligomer ELISA kit (KIT29), BioPorto Diagnostics, Denmark. Complement levels such as that of C3 and C4 were detected using a Nephelometer (BN ProSpec, Dade Behring, Germany). Anti-dsDNA antibodies were detected using indirect immunofluroscence method (Euroimmun Lubeck, Germany), where Crithidia luciliae was used as a substrate. The laboratory was blinded to the disease status of the patients and their visceral involvement, and a double-blinded study was conducted on the autoantibody-positive samples.
Results
Details of the demographic charateristics in the SLE patients included in this study, at the time of evaluation, are shown in Table 1. Age of onset of the disease was observed to be 14-47 (25.7 ± 8.3) years and the age of evaluation was 17-49 (29.7 ± 8.1) years. A total of 45/60 (75%) SLE patients had LN, and the remaining 15/60 patients (25%) who did not show renal manifestations were grouped as non-LN. The mean duration of the disease was found to be 43.2 ± 16.9 months. The average number of American College of Radiology Rheumatology (ACR) criteria met by the SLE patients at evaluation was 5.3 ± 1.3, and the SLEDAI scores ranged between 4 and 30 (14.6 ± 4.4). The cutoff for the CIC levels was 20 units/ml. All SLE patients included in this study showed elevated CIC levels (>100 units/ml). Anti-dsDNA positivity was slightly higher (65.7%) among the anti-C1q-positive patients compared to the anti-C1q-negative patients (60%).

The assay cutoff for anti-C1q antibody positivity was set at 50 μg/ml; levels of anti-C1q antibodies above this value were considered positive. The measuring range varied between 3.72 and 100 μg/ml. Of all the SLE patients tested, 35/60 patients (58.3%) showed a high prevalence of anti-C1q antibodies with mean ± SD values of 80.9 ± 17.8. Renal histopathological findings in LN patients showed that 10/45 patients (22.2%) had mesangial proliferative glomerulonephritis (MPGN, Class I and II), 23/45 patients (51.1%) had diffuse proliferative glomerulonephritis (DPGN, Class IV), 4/45 patients (8.9%) had membranous lupus nephritis (Class V), and none of the patients had focal proliferative glomerulonephritis (FPGN, Class III). The remaining eight patients did not give their consent for renal biopsy. Among the LN patients, 27 (60%) were anti-C1q positive. Anti-C1q antibody positivity was the highest among DPGN patients (59.3%), followed by the MPGN group (18.5%).
The normal levels of C3 ranged between 90 and 180 mg/dL, while those for C4 ranged between 15 and 40 mg/dL. The detection range for the serum MBL levels was 5-4000 ng/ml. The low MBL level range was <500 ng/ml and high MBL level range was >500 ng/ml. The distribution of the anti-C1q antibodies with complement component levels in SLE patients among LN and non-LN groups is shown in Table 2. Reduced levels of C3 and C4 individually, as well as in both the C3 and C4 levels together, were seen at a higher percentage in patients with anti-C1q antibodies. LN patients showed a higher percentage of low MBL levels (61.1%) among the anti-C1q negatives compared to the anti-C1q positives (55.6%). The non-LN group had a higher percentage of low MBL levels (87.5%) among the anti-C1q positives compared to the anti-C1q negatives (57.1%).

The distribution of patients based on their disease activity as per the SLEDAI scores is as shown in Figure 1. Overall, it was observed that 51.7% of the patients showed moderate disease (SLEDAI 8-18). Among the anti-C1q positives who had moderate disease, 44.5% of the patients were LN compared to 37.5% non-LN. Among the anti-C1q negatives with moderate disease activity, 85.7% were non-LN and only 55.6% patients were LN. Among the anti-C1q negatives, none of the patients belonging to the non-LN group had severe disease (SLEDAI >18).
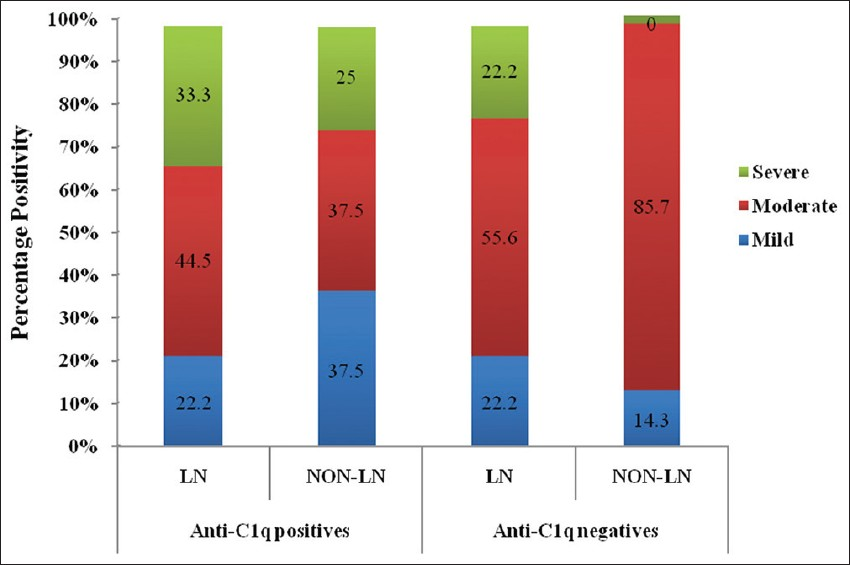
- Distribution of anti-C1q antibodies and clinical severity
Discussion
Higher prevalence of anti-C1q antibodies of around 80-100% had been reported in LN patients by various groups.[91617] A comparatively lower prevalence of anti-C1q antibodies ranging between 55 and 60% was also reported in LN patients.[18–22] Previous studies reported 20-66% anti-C1q antibody prevalence in SLE patients.[23–26] These discrepancies may be due to differences in the patient populations studied, as well as in the specificity and sensitivity of the anti-C1q ELISA and the variations in the commercially available kits used. Our study showed an overall incidence of 58.3% for anti-C1q antibodies among the SLE patients studied where the LN patients had 60% anti-C1q positivity. These findings were in accordance with the earlier studies.[18–26]
The prevalence of anti-C1q antibodies among the non-LN patients in this study was 53.3%, which was in accordance with the 41.5-55.5% prevalence reported in the earlier studies, including our previous study.[172127] Our study showed a slightly higher incidence of anti-C1q antibodies (60%) in LN patients compared to non-LN patients (53.3%). This finding was similar to the studies reported by Zang et al. and Katsumata et al.[2122] It was suggested that the circulating anti-C1q antibodies may bind to the C1q deposits in the kidneys of LN patients and this consumption of serum anti-C1q antibodies by binding to C1q-containing immune complexes could be responsible for the lack of significant difference among LN and non-LN patients.[24] It was also reported that although a high prevalence of anti-C1q antibodies correlated with proliferative LN, the predictive value of 27-68%, in the presence of anti-C1q antibodies, among LN patients was too low to reliably identify them as LN, and that up to 46% renal flares occurred in patients who did not develop anti-C1q antibodies.[92829] These findings suggested that anti-C1q antibodies were not useful as possible biomarkers for LN in SLE and our findings supported the same. High prevalence of anti-C1q antibodies in SLE might have important consequences for possible renal manifestations as well for the understanding of pathogenic mechanisms.
Complement components such as C3 and C4 were usually low in active SLE.[3] Anti-C1q antibodies were associated with reduced levels of both C3 and C4 together, as well as individually, in LN patients, indicating their role in immune complex clearance via the classical pathway. Structural as well as functional similarities in C1q and MBL prompted us to study the association between anti-C1q antibodies and MBL. A higher percentage of low MBL levels in anti-C1q positives among the non-LN group were noted in our study, which suggested that the anti-C1q antibodies did not recognize the MBL pathway for immune clearance. Similar findings were also reported indicating that lowered serum MBL levels could be attributed to various different factors such as mutated genes or anti-MBL antibodies.[30] Our study did not show a correlation between anti-C1q-positive patients and their SLEDAI scores. This was similar to the recent reports where anti-C1q antibodies were not associated with SLEDAI scores for disease activity or for the presence of dsDNA antibodies in them.[1726]
Source of Support: Nil
Conflict of Interest: None declared.
References
- The role of mannose-binding lectin in systemic lupus erythematosus. Clin Rheumatol. 2008;27:413-9.
- [Google Scholar]
- Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501-37.
- [Google Scholar]
- Antibodies against complement system in SLE and their diagnostic utility. Curr Rheumatol Rev. 2009;5:58-63.
- [Google Scholar]
- Cutting edge: C1q protects against the development of glomerulonephritis independently of C3 activation. J Immunol. 1999;162:5676-9.
- [Google Scholar]
- Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56-9.
- [Google Scholar]
- Ststemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227-324.
- [Google Scholar]
- IgG autoantibodies against C1q are correlated with nephritis, hypocomplementamia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230-4.
- [Google Scholar]
- Anti-C1q antibodies in nephritis: Correlation between titers and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:444-8.
- [Google Scholar]
- Changes in antibodies to C1q predict renal relapses in systemic lupus erythematosus. Am J Kidney Dis. 1995;26:595-601.
- [Google Scholar]
- Subclass distribution of IgA and IgG antibodies against Clq in patients with rheumatic diseases. Scand J Immunol. 1995;41:391-7.
- [Google Scholar]
- Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725-5.
- [Google Scholar]
- Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630-40.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241-50.
- [Google Scholar]
- High prevalence of anti-C1q antibodies in biopsy proven active lupus nephritis. Nephrol Dial Transplant. 2006;21:3115-21.
- [Google Scholar]
- Anti-C1q antibodies antedate patent active glomerulonephritis in patients with systemic lupus erythematosus. Arthritis Res Ther. 2009;11:R87.
- [Google Scholar]
- Anti-C1q autoantibodies in lupus nephritis: prevalence and clinical significance. Ann N Y Acad Sci. 2005;1050:193-200.
- [Google Scholar]
- Detection of anti-C1q antibodies and anti-C1q globular head domain antibodies in sera from Chinese patients with lupus nephritis. Mol Immunol. 2009;46:2178-82.
- [Google Scholar]
- Anti-C1q antibodies and IgG subclass distribution in sera from Chinese patients with lupus nephritis. Nephrol Dial Transplant. 2009;24:172-8.
- [Google Scholar]
- Anti-C1q antibodies are associated with systemic lupus erythematosus disease activity and lupus nephritis in northeast of China. Clin Rheumatol. 2011;30:967-73.
- [Google Scholar]
- Anti-C1q antibodies are associated with systemic lupus erythematosus global activity, but not specifically with nephritis: A controlled study of 126 consecutive patients. Arthritis Rheum. 2011;63:2436-44.
- [Google Scholar]
- Autoantibodies against C1q in Systemic Lupus Erythematosus are antigen-driven. J Immunol. 2009;183:8225-31.
- [Google Scholar]
- Correlation between the renal C1q deposition and serum anti-C1q antibody in lupus nephritis. Asian Pac J Allergy Immunol. 2002;20:223-7.
- [Google Scholar]
- Anti-C1q antibodies: association with nephritis and disease activity in systemic lupus erythematosus. J Clin Lab Anal. 2009;23:19-23.
- [Google Scholar]
- Autoantibodies against protective molecules--C1q, C-reactive protein, serum amyloid P, mannose-binding lectin, and apolipoprotein A1: Prevalence in systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1108:227-39.
- [Google Scholar]
- Fc γ RIIA Genotypes and Its Association with Anti-C1q Autoantibodies in Lupus Nephritis (LN) Patients from Western India. Autoimmune Dis. 2010;2010:470695.
- [Google Scholar]
- Are laboratory tests useful for monitoring the activity of lupus nephritis? A 6-year prospective study in a cohort of 228 patients with lupus nephritis. Ann Rheum Dis. 2009;68:234-7.
- [Google Scholar]
- Human autoantibodies against C1q: lack of cross reactivity with collectins mannan-binding protein, lung surfactant protein A and bovine conglutinin. Scand J Immunol. 1996;43:314-20.
- [Google Scholar]







