Translate this page into:
Antiglomerular Basement Membrane Disease Combined with IgA Nephropathy
Address for correspondence: Dr. Yashita Gupta, Department of Histopathology, Apollo Hospitals, Hyderabad, Telangana, India. E-mail: yashigupta@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Anti-glomerular basement membrane (GBM) glomerulonephritis is one manifestation of a disease process that can involve the lung as well as the kidney and is secondary to vascular injury mediated by antibodies directed against the GBM.[1] Anti-GBM disease is usually diagnosed by high titers of immunoglobulin G (IgG) autoantibodies to the non-collagenous (NC1) domain of α3 (Type IV) collagen in the circulation and/or renal biopsy.[2] Anti-GBM antibodies have been identified to coexist in a proportion of patients with antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis usually pANCA and infrequently in patients with membranous nephropathy.[3] We hereby report a case of a rare association of anti-GBM disease with IgA nephropathy.
A 30-year-old man presented with the complaint of sudden onset facial puffiness and swelling of feet accompanied by oliguria and later anuria. On examination, he had pallor, 1+ pitting edema in the lower extremities and facial puffiness. His blood pressure was in the normal range. Serum creatinine was increased to 13.13 mg/dL. Urinalysis showed 10–12 red blood cells per high-power field and 3+ proteinuria. Urine protein to creatinine ratio was 2.5 g/g. Renal ultrasound showed normal renal size and bilateral increased echogenicity. Serologic test results were strongly positive for IgG anti-GBM antibody by enzyme-linked immunosorbent assay; serum C3 and C4 levels were normal. Other serologic tests for antinuclear antibody, antidouble-stranded DNA antibody, rheumatoid factor, cryoglobulinemia, hepatitis B and C, and ANCA were negative. Kidney biopsy was performed.
Light microscopic evaluation showed a core of renal cortex with adjoining medulla with nine glomeruli and two arteries. One glomerulus was partially sclerosed. All nine glomeruli showed crescents, five cellular and four fibrocellular crescents. The underlying tuft showed mild to moderate PAS positive mesangial widening with variable increase in mesangial cellularity. Endocapillary hypercellularity was also seen. There was no evidence of segmental sclerosis. The tubulointerstitial compartment showed mild tubular injury with hyaline and granular casts. Moderate tubular atrophy was seen involving about 35–40% of the cortex studied. The interstitium was widened and fibrosed around the atrophic tubules with moderate infiltrate of lymphocytes, few plasma cells, and occasional neutrophils. The blood vessels included were normal [Figures 1 and 2].

- H and E, 20× view shows two glomeruli with crescents, underlying tuft being cellular. There is moderate lymphocytic infiltrate in tubulointerstitial compartment and blood vessel included appears normal
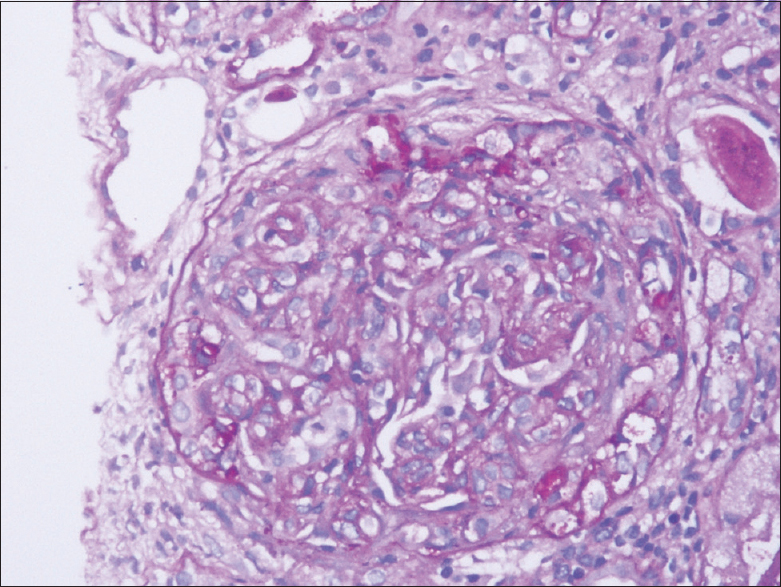
- PAS, 40× view shows single glomerulus with crescent and underlying tuft showing mesangial and endocapillary hypercellularity
Tissue submitted for immunofluorescence studies contained seven glomeruli, which showed linear GBM staining with IgG (3-4+) and mesangial granular staining with IgA (3-4+), C3c (2+); and kappa (3+) and lambda (3+) light chains [Figures 3 and 4].
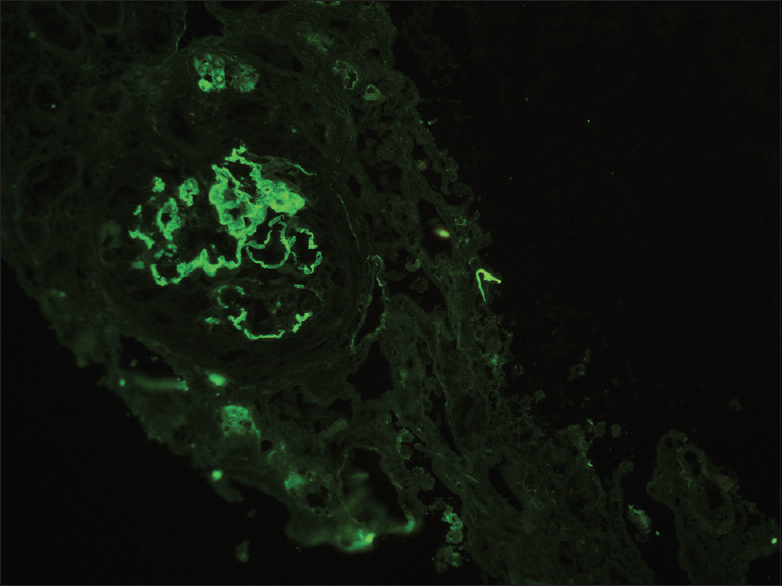
- Immunofluorescence with IgG antibody shows linear IgG staining along glomerular capillary basement membranes with 3–4+ intensity
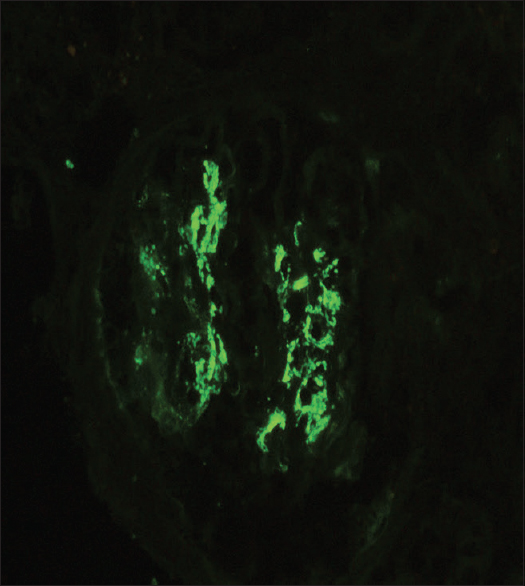
- Immunofluorescence with IgA antibody shows granular staining in mesangium with 3–4+ intensity
Based on the above findings, the diagnosis of anti-GBM disease with IgA nephropathy was confirmed. The patient received intravenous steroid therapy and plasmapheresis, followed by intravenous cyclophosphamide and steroid for maintenance therapy. On last follow-up, the patient was fairly well with decrease in anti-GBM antibody to normal level but serum creatinine was still at 3.0 mg/dL with persistent hematuria.
Few cases have been reported in past showing anti-GBM disease with linear IgA deposits along GBMs,[4] but anti-GBM disease combined with IgA granular deposition in the mesangial area is rarely discussed. Before giving a diagnosis of associated primary IgA nephropathy other differential diagnosis that could reveal IgA granular deposits in the mesangium under immunofluorescence should be excluded, such as IgA-dominant acute postinfectious glomerulonephritis, Henoch–Schönlein purpura, and HBV-glomerulonephritis.[5]
First report of anti-GBM disease and mesangial IgA deposits was described in 1998 in a 12 year postrenal transplant recipient. As per this report, the IgA deposits may promote the release of inflammatory mediators, resulting in conformational changes of the glomerular basement membrane and exposure of the GBM antigens, thus facilitating anti-GBM antibody generation.[6]
Another case report by Wang et al., highlighted the possibility of increased antigen synthesis, exposure of cryptic epitopes, or capping and shedding of antigen antibody complexes.[7]
In previous reports published of this rare entity it was found that as compared to classical anti-GBM disease, the prognosis of anti-GBM disease associated with IgA deposits is poor. Therefore, it is important to identify this condition histopathologically with careful assessment of pattern of immunofluorescence staining which might give an insight into its pathogenesis in further studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Sanjay Maitra, Nephrologist.
References
- Silva's Diagnostic Renal Pathology 2017:243-64.
- Concurrent antiglomerular basement membrane antibody and immune complex mediated glomerulonephritis. Am J Clin Pathol. 1982;78:381-6.
- [Google Scholar]
- IgA variant of anti-glomerular basement membrane glomerulonephritis associated with pulmonary hemorrhage and microangiopathic hemolytic anemia. Ren Fail. 2012;34:657-60.
- [Google Scholar]
- IgA-dominant postinfectious glomerulonephritis: A new twist on an old disease. Nephron Clin Pract. 2011;119:c18-25. discussion c26
- [Google Scholar]
- Recurrence of anti-GBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol. 1998;49:124-8.
- [Google Scholar]
- Mesangial IgA deposits indicate pathogenesis of anti-glomerular basement membrane disease. Mol Med Rep. 2012;5:1212-4.
- [Google Scholar]






