Translate this page into:
Approach to Diagnosis and Management of Pediatric Hypertension in an Outpatient Setting
Corresponding author: Rahul Chanchlani, Department of Pediatrics, McMaster University, Hamilton, Ontario, Canada. E-mail: chanchlr@mcmaster.ca
-
Received: ,
Accepted: ,
How to cite this article: Khullar S, Asaithambi A, Pais P, Chanchlani R. Approach to Diagnosis and Management of Pediatric Hypertension in an Outpatient Setting. Indian J Nephrol. doi: 10.25259/IJN_385_2024
Abstract
Pediatric hypertension (HTN) is a public health concern with significant possible long-term adverse outcomes. This review is a comprehensive guide for pediatricians, nephrologists, and trainees, focusing on the latest approaches for HTN diagnoses in children and highlighting the importance of accurate blood pressure measurement techniques. We also explore current classification systems and offer evidence-based HTN management strategies tailored to pediatric patients. Lifestyle modifications are the recommended first-line interventions, including dietary changes, physical activity, and weight management. Pharmacological treatments are for severe cases or when lifestyle modifications are insufficient. The guidelines provide an overview of commonly prescribed antihypertensive medications, potential complications associated with untreated HTN, including target organ damage and increased cardiovascular risk in adulthood, and the importance of early recognition and intervention. This review aims to help healthcare professionals thoroughly understand pediatric HTN to improve diagnosis, treatment, and long-term outcomes.
Keywords
Blood pressure in children
Childhood hypertension
Hypertension classification
Hypertension diagnosis
Hypertension management
Hypertension risk factors
Hypertension treatment strategies
Pediatric hypertension
Pediatric hypertension complications
Introduction
Hypertension (HTN) is one of the most common causes of preventable cardiovascular (CV) disease.1 The pediatric elevated blood pressure (BP) pooled prevalence [SBP and/or DBP ≥90th percentile but <95th percentile (for age, sex, and height) or ≥120/80 mmHg] globally and in India is 9.67% and 10.0%, respectively.2,3 The HTN prevalence in children ≤19 years is 4%,2,3 and strong evidence suggests that childhood BP tracks into adulthood and is associated with premature CV and kidney disease.4–8 Hence, early detection of HTN in children is essential. This review aims to provide an approach to outpatient pediatric HTN for pediatricians and trainees. It discusses the investigation and management of HTN in children using 2 clinical cases of primary and secondary pediatric HTN.
A 10-year-old female presented to the pediatric clinic with persistent headaches for the past few weeks. Her mother reported her having occasional dizziness. The headaches had no associated triggers, such as physical activity or changes in position. The patient had a 27.5 kg/m2 BMI and a 128/86 mmHg BP reading. Her mother had a family history of HTN, with her grandmother being diagnosed in her early 40s.
An 8-year-old male, presented to the pediatric clinic with elevated BP readings noted during a routine school health screening. His parents also reported him having frequent nosebleeds over the past few months. His maternal aunt was diagnosed with polycystic kidney disease in her 30s. His BP was 124/83 mmHg during his clinic assessment.
Measuring blood pressure in pediatric populations
Office BP: Most international guidelines rely on office BP measurement for HTN diagnosis and management. Having standardized and reliable BP measurements is crucial for HTN diagnoses; unfortunately, there may be challenges to measuring BP in a pediatric patient. Ideally, BP should be measured with the child seated calmly for at least 5 minutes with feet on the ground and their arm resting at the level of their heart (Figure 19 and refer to https://www.broadcastmed.com/cardiology/3979/videos/blood-pressure-measurement-in-children for video example). BP can be assessed using auscultatory techniques with an aneroid sphygmomanometer,4,10 or by validated and periodically calibrated oscillometric devices, ensuring that the cuff covers 80% to 100% of the arm circumference.11,12 For children, the cuff bladder width should be at least 40% of the arm circumference measured halfway between the olecranon and acromion. In neonates, a cuff width-to-arm circumference ratio between 0.45 and 0.70 is recommended.8,13
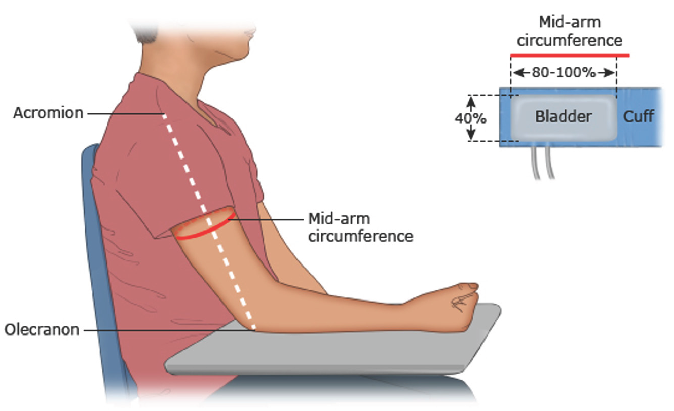
- Pediatric blood pressure measurement - cuff sizing and position.9
Oscillometric devices are accepted screening tools for children. Any elevated BP reading should be confirmed using the auscultatory method, which is better for predicting target organ damage. If the BP is initially elevated, two additional readings should be taken, and the average must be used as the final record. HTN should only be diagnosed in an office setting based on readings from three separate consecutive occasions.14,15 BP measurements using the forearm or wrist are not recommended for children.15 Table 1 shows the definition and HTN classification in children.8,16,17
| 2017 American academy of pediatrics8 | 2020 hypertension Canada17 | 2016 European Society of Hypertension16 | ||||
|---|---|---|---|---|---|---|
| Blood pressure category | Children aged 1 to <13 years | Children aged ≥13 years | Blood pressure category | <16 years | ≥16 years | |
| Normal BP | SBP and DBP <90th percentile | SBP <120 and DBP <80 mmHg | BP < 95th percentile for age, sex and height or in 6-11 year old children BP <120/80 or in 12-17 year old children BP <130/85 | Normal BP | <90th percentile | SBP 120-129 and DBP 80-84 |
| Elevated BP | SBP and DBP ≥90th percentile to <95th percentile or 120/80 mmHg to <95th percentile (whichever is lower) | SBP 120 to 129 and DBP <80 mmHg | ____ | High normal BP | ≥90th-95th percentile | SBP 130-139 and/or DBP 85-89 |
| Stage 1 Hypertension | SBP and DBP ≥95th percentile to <95th percentile +12 mmHg or 130/80 to 139/89 mmHg (whichever is lower) | BP 130/80 to 139/89 mmHg | SBP and DBP ≥95th percentile to <95th percentile + 12 mmHg | Stage 1 Hypertension | 95th-99th percentile and 5 mmHg | SBP 140-159 and/or DBP 90-99 |
| Stage 2 Hypertension | SBP and DBP ≥95th percentile +12 mmHg or ≥140/90 mmHg (whichever is lower) | BP ≥140/90 mmHg | SBP and DBP ≥95th percentile + 12 mmHg | Stage 2 hypertension | >99th percentile and 5 mmHg | SBP 160-179 and/or DBP 100-109 |
SBP: Systolic blood pressure, DBP: Diastolic blood pressure, BP: Blood pressure, HTN: Hypertension
Ambulatory BP monitoring: Office BP readings have several drawbacks, including the risk of missing white coat HTN (WCH), where office BP is ≥95th percentile but ambulatory BP is normal, or masked HTN (MH), where office BP is normal but ambulatory BP is ≥95th percentile.18 ABPM overcomes these issues and shows better association with target organ damage; thus, it is recommended.15 Based on clinic and ABPM, there are 4 phenotypes of HTN according to American Heart Association 2022 guidelines, including normal BP, white coat HTN, masked HTN, and ambulatory HTN19 [Table 2].
| Category | Clinic SBP or DBP* | Mean ambulatory SBP or DBP |
|---|---|---|
| Normal BP | <95th percentile 9 (<13 years) <130/80 (≥13 years) |
95th percentile or adolescent cut points* (<13 years) <125/75 mmHg 24-h and <130/80 mmHg wake and <110/65 mmHg sleep (≥13 years) |
| WCH | ≥95th percentile (<13 years) ≥130/80 (≥13 years) | |
| Masked hypertension | <95th percentile (<13 years) <130/80 (≥13 years) |
≥95th percentile or adolescent cut points* (<13 years) ≥125/75 mmHg 24-h or ≥130/80 mmHg wake or ≥110/65 mmHg sleep (≥13 years) |
| Ambulatory hypertension | ≥95th percentile (<13 years) ≥130/80 (≥13 years) |
Home BP monitoring: Home blood pressure monitoring (HBPM) is regularly measuring and recording BP levels at home using a digital or manual BP monitor. This practice allows patients to track their BP, and it has gained more popularity during COVID-19.20 In addition to providing a more longitudinal BP assessment over ABPM, HBPM is cost-effective and well-tolerated.21,22 HBPM is not recommended for diagnosing HTN in pediatric patients.8,16 Limitations of HBPM include reporting bias, inconsistent measurement times, and lack of validated devices/cuff sizes for pediatric patients.16
Frequency of BP monitoring: The American Academy of Pediatrics guidelines recommend annual BP measurements for children aged ≥3 years, with more frequent checks for obese patients, those on medications that increase BP, or with conditions like renal disease, coarctation, or diabetes.15 Children < 3 years should have regular BP measurements if they have congenital heart disease, recurrent urinary tract infections, urological malformations, solid organ transplants, bone marrow transplants, malignancies, neurofibromatosis, tuberous sclerosis, or sickle cell disease. Newborns who are small for gestational age, premature (less than 32 weeks), have very low birth weight, or umbilical arterial catheterization also require regular checks.15 The HTN Canada guidelines recommend regular BP measurement for children ≥3 years but do not specify the frequency of screening.
Primary versus secondary HTN
HTN without a clearly defined etiology is considered primary. Primary HTN is typically an exclusive diagnosis, occurring more frequently in overweight/obese children > 6 years of age with a family history of HTN.8
Secondary HTN is due to an identifiable underlying cause. It must be suspected in children < 6 years of age with HTN or at any age with severe HTN. Secondary HTN pooled prevalences among the United States children undergoing HTN evaluation in a hospital outpatient setting and primary care/community settings were 8%23 and 3.7%, respectively. The highest secondary HTN prevalence (20%) was observed in HTN clinics.23 The common causes of HTN classified according to age group have been listed in Table 3.
| Renal | Others | |
|---|---|---|
| Newborn |
Renal parenchymal disease: Autosomal recessive and dominant polycystic kidney disease Renal dysplasia Renovascular disease (such as thrombosis of renal artery or vein) |
Cardiac causes such as coarctation of aorta Bronchopulmonary dysplasia Post ECMO |
| Children |
Renal parenchymal disease: Acute glomerulonephritis Hemolytic uremic syndrome Urinary tract infections Reflux nephropathy Renovascular disease (renal artery stenosis/renal vein thrombosis) |
Coarctation of aorta Monogenic HTN Wilms tumor Neuroblastoma Primary HTN |
| Adolescents |
Renal parenchymal disease Renovascular disease (similar causes for children) |
Primary HTN Coarctation of aorta Endocrine causes, including Cushing’s syndrome, hyperthyroidism, hypothyroidism, pheochromocytoma Drug-induced including glucocorticoids, calcineurin inhibitor, sympathomimetics (salbutamol, aminophylline), growth hormone, decongestants, stimulants, antidepressants, hormonal contraceptives, substance abuse (cocaine, MDMA/ecstasy) |
HTN: Hypertension, ECMO: Extracorporeal membrane oxygenation, MDMA: 3,4-methylenedioxymethamphetamine
Clinical symptoms of HTN
Primary HTN in children is usually mild or moderate, asymptomatic (stage 1 or less) with insidious onset. Detection is often incidental during routine medical checkups. Primary HTN risk factors may be modifiable (increased BMI, stress, reduced physical activity, and high salt intake) or non-modifiable (family history, race, and perinatal history) as shown in Table 4. Our case (10-year-old female) highlights some of these risk factors, including elevated BMI and a family history of HTN.
| History | Findings |
|---|---|
| Perinatal history |
Maternal HTN, maternal diabetes Birth related - Low birth weight, preterm birth23 Oligohydramnios Pre-eclampsia |
| Family history in parents or grandparents | HTN or cardiovascular disease |
| Lifestyle |
Unhealthy weight gain Sedentary lifestyle Lack of physical activity Sleep apnea Excessive salt intake Consumption of high-fat foods Consumption of sugary beverages Infrequent consumption of fruits, vegetables, and low-fat dairy products |
HTN: Hypertension
Secondary HTN, if severe (Stage 2 or higher), may present with symptoms that indicate the causes, such as headache, vomiting, abdominal pain, epistaxis, palpitations, or flushes. It can also have etiology specific symptoms as listed in Table 5. Severe HTN may present as hypertensive encephalopathy (altered sensorium, visual disturbances, seizures, or rarely focal neurological deficits) or as congestive heart failure. The causes and clinical features of secondary HTN in children have been presented in Table 5. Our case (8-year-old male) had frequent nose bleeds and family polycystic kidney disease, suggesting secondary HTN.
| Cause | Relevant history and findings | |
|---|---|---|
| Renal parenchymal disease | Reflux nephropathy | History of UTI, abnormal upper or lower urinary tract imaging |
| Post infectious GN | Gross hematuria, edema, preceding infection, low C3 | |
| IgA vasculitis associated nephritis | Purpuric rash, hematuria, edema | |
| Lupus nephritis | Edema, hematuria, malar rash, joint pain, oral ulcer, photosensitivity, Raynaud’s phenomenon | |
| Hemolytic uremic syndrome | Hemolytic anemia, thrombocytopenia, hematuria, renal failure | |
| Acute tubulointerstitial nephritis | Sterile pyuria, dysuria, fatigue | |
| Nephrotic syndrome | Edema | |
| Chronic glomerulonephritis | Proteinuria, hematuria, elevated serum creatinine | |
| Autosomal dominant polycystic kidney disease, Autosomal Recessive polycystic kidney disease | Palpable kidneys, USG showing renal cysts, family history of cysts | |
| Chronic kidney disease | Growth retardation, previous history of UTI or renal issues | |
| Acute urinary obstruction |
Pelvic ureteric junction obstruction Ureteric/bladder calculi |
Flank pain, renal mass, palpable bladder, trauma |
| Renovascular disease |
Renal artery stenosis Arteritis Renal artery or venous thrombosis |
Neurofibromas, café au lait (NF), renal bruit Pulse discrepancy, claudication, Raynaud phenomenon Gross hematuria, renal mass, umbilical catheterisation |
| Cardiovascular | Coarctation of aorta, hypoplastic abdominal aorta syndrome | Decreased or absent femoral pulses, discrepancy in four limb BP – higher BP in upper limbs compared to lower limbs, Systolic murmur |
| Central nervous system |
Increased intracranial pressure Spinal injury, Gullian Barre syndrome Neurofibromatosis Tuberous sclerosis |
Head trauma, intracranial bleed, meningitis, bulging fontanelle and increase head circumference History of trauma, limb paralysis, loss of bowel or bladder control Café-au-lait spots Adenoma sebaceum |
| Endocrine |
Pheochromocytoma Hyperthyroidism Hypothyroidism Cushing’s syndrome Congenital adrenal hyperplasia |
Flushing, palpitations, headache, diaphoresis Weight loss, tremor, heat intolerance, thyromegaly and exophthalmos Weight gain, constipation, dry skin, cold intolerance Obesity, moon-faced, acne, hirsutism, striae Ambiguous genitalia, virilisation |
| Monogenic HTN | E.g., Apparent mineralocorticoid excess, Gordon’s syndrome | Failure to thrive, muscle weakness, reduced renin levels, early onset, family history |
| Tumor |
Wilms tumor Neuroblastoma |
Gross hematuria, abdominal mass |
| Medications and Substance abuse |
Glucocorticoids, calcineurin inhibitor, sympathomimetics (salbutamol, aminophylline) growth hormone, erythropoietin, phenylephrine in decongestants, stimulants, antidepressants, hormonal contraceptives Substance Abuse (cocaine, MDMA/Ecstasy) |
|
HTN: Hypertension, UTI: Urinary tract infection, GN: Glomerulonephritis, USG: Ultrasonography, MDMA: 3,4-methylenedioxymethamphetamine
Renovascular HTN
A high index of suspicion is useful for an early diagnosis of renovascular HTN. Examination and history can provide various clues [Box 1].
Monogenic HTN
Monogenic HTN disorders are a distinct group of diseases causing renin–angiotensin–aldosterone system dysregulation, as listed in Table 6.24 The hallmarks of monogenic forms of HTN are suppressed plasma renin, inappropriate distal sodium absorption, and volume expansion. While early-onset refractory HTN, hypokalemia, or hyperkalemia and family history are classical, phenotypic heterogeneity can occur. Monogenic causes should be suspected in the absence of renal parenchymal, renovascular, endocrine, or exogenous causes, irrespective of age or family history. Genetic diagnosis of these monogenic disorders is important since therapy is specific to the underlying molecular abnormality. For a detailed approach to monogenic HTN, refer to Table 6.
| Low renin levels | Low aldosterone levels |
Liddle syndrome Congenital adrenal hyperplasia Apparent mineralocorticoid excess Gellers syndrome |
| Normal aldosterone levels | Gordon syndrome (pseudo hypoaldosteronism type II) | |
| High aldosterone levels |
Familial hyperaldosteronism type I (glucocorticoid-remediable aldosteronism) Familial hyperaldosteronism type II Familial hyperaldosteronism type III Familial hyperaldosteronism type IV |
HTN: Hypertension
Key investigations to evaluate childhood HTN
Keeping in mind the cause of HTN based on history and clinical examination, the stepwise evaluation for HTN has been elaborated in Box 2 and Table 7 outlines a detailed evaluation approach for secondary causes of hypertension.
| Clinical diagnosis | Confirmatory evaluation |
|---|---|
| Glomerulonephritis |
Serum C3, C4, ASO Autoantibodies (ANA/anti dsDNA/ANCA) Renal biopsy |
| Reflux nephropathy |
Micturating cystourethrogram Nuclear scan (DMSA/MAG3) |
| Renovascular disease |
Plasma renin/aldosterone levels Kidney doppler (low sensitivity) CT/MR renal angiography (>95% sensitive) Digital subtraction angiography – Gold standard |
| Pheochromocytoma |
Urine and plasma metanephrines MIBG scan CT/MRI abdomen Arteriography and caval catecholamine sampling |
| Hyperthyroidism or hypothyroidism | Thyroid stimulating hormone, free T4 and free T3 |
|
Endocrine causes Cushings syndrome Primary aldosteronism |
Overnight dexamethasone suppression test, 24 hour urinary free cortisol Plasma aldosterone renin ratio |
| Coarctation of aorta | Echocardiogram |
| Neuroblastoma | Vanillylmandelic acid (VMA), homovanillic acid (HMA) |
| Monogenic Hypertension |
Aldosterone and renin levels Genetic testing |
ASO: Antistreptolysin O, ANA: Antinuclear antibody, dsDNA: Double-stranded deoxyribonucleic acid, ANCA: Anti-neutrophil cytoplasmic antibody, DMSA: Dimercaptosuccinic acid, MAG3: Mercaptuacetyltriglycine, CT: Computed tomography, MR: Magnetic resonance, MIBG: Metaiodobenzylguanidine, MRI: Magnetic resonance imaging
Consequences of pediatric HTN
Hypertensive children are likely to become hypertensive adults
During early childhood, individual BP levels can vary between measurements. However, around 8–9 years of age, BP levels within individuals tend to track along the same percentile. Evidence suggests that primary pediatric HTN predicts adult HTN, with a tracking coefficient of at least 0.4.25 Children with higher BP levels are more likely to carry them into adulthood. This persistence raises the risk for subsequent cardiovascular diseases (CVD) in adulthood.25
Target organ damage in children with HTN
Strong evidence backs the association between pediatric HTN and adverse subclinical cardiovascular outcomes or target organ damage. In adults, these subclinical outcomes are consistently linked to an increased risk of cardiovascular events. In a systematic review of 12,252 studies, children with ambulatory HTN had an elevated LVH risk (odds ratio, 4.69 [95% CI, 2.69-8.19]), left ventricular mass index, pulse wave velocity, carotid intima-media thickness, and retinopathy and albuminuria compared with normotensive children.26
Subclinical markers related to vascular structures include carotid intima-media thickness (CIMT), arterial stiffness (measured using pulse wave velocity), and endothelial function (assessed through brachial flow mediated dilation).26 Microvascular changes associated with BP have been observed in childhood, including abnormal central retinal arteriolar and venular diameters. Microvascular dysfunction is one proposed mechanism linking higher BP levels to subtle preclinical cognitive function changes in adolescents.
Future Kidney and CV outcomes of pediatric HTN
In a large Israeli military recruits cohort, adolescent HTN (16–19 years old) was associated with an increased long-term kidney failure risk (adjusted hazard ratio 1.98, 95% CI 1.42–2.77), irrespective of BMI status or HTN severity.27
There are recent data showing a strong association between HTN and future CV outcomes. In a recent population-based study in Ontario using health administrative databases with a 13.6 (7.8-19.5) years median (IQR) follow-up, major adverse CV event incidences in children with HTN and controls were 4.6 per 1000 person-years vs. 2.2 per 1000 person-years (hazard ratio, 2.1; 95% CI, 1.9-2.2), respectively. Children with HTN were at higher associated risk of stroke, myocardial infarction, unstable angina, coronary intervention, and congestive heart failure compared with non-hypertensive controls.28 In a large prospective study of the International Childhood Cardiovascular Cohort (i3C) Consortium – published in NEJM in 2022, researchers assessed whether cardiovascular risk factors measured in childhood (ages 3–19) were linked to cardiovascular events in adulthood over an average follow-up period of 35 years. They analyzed factors like BMI, SBP, cholesterol, triglycerides, and youth smoking. Outcomes included both fatal and nonfatal cardiovascular events. This study found that childhood risk factors significantly predicted CV events in adulthood, even when measured decades later. Specifically, smoking increased the risk of fatal CV in adult ages by 60%. The combined-Risk Z score (which incorporated childhood BMI, systolic BP, cholesterol, triglycerides, and smoking status) showed that each unit increase was associated with a 2.71-fold higher risk for fatal CV events in adulthood. Individual risk measures, such as high systolic BP and elevated cholesterol, found that each unit increase in the z-score raised the hazard for adult CV events by 1.3 to 1.6 times, respectively.29 These findings emphasize CV risk factor management from an early age, as it may significantly reduce the likelihood of adverse CV events later. Another study demonstrated the direct and indirect association between childhood risk factors and adult CVD, with the largest direct effect seen for BMI and LDL-C. The results highlighted that childhood BMI and LDL-C had significant direct effects on later CVD risk, with an incidence rate ratio (RR) of 1.18 for BMI and 1.16 for LDL-C per one standard deviation increase.30 The study emphasized the importance of early-life interventions targeting these risk factors—especially BMI— as childhood influences on CVD risk are not fully mitigated by later risk management.
Non-pharmacological management: Optimal BP thresholds are unknown for pediatric populations, but HTN management aims to minimize future cardiovascular and renal disease risks. Current strategies for pediatric HTN management are primarily at the patient level rather than population-based.25 Non-pharmacological pediatric HTN management involves lifestyle and behavioral changes. Key strategies include:
Dietary Modifications:
Reduced Salt Intake: There is substantial evidence indicating better BP control by lowering dietary sodium in children, showing a dose-dependent effect.31,32 Two pediatric meta-analyses, encompassing 966 and 58,531 patients, demonstrated significant BP reductions by reducing sodium intake in the diet (approximately 1 mm Hg). This correlation is more pronounced in overweight children and those with low potassium intake.33,34 The high sodium content in processed foods makes sustainable intake difficult.35 While sodium reduction targets for children remain unclear, the National Academies of Sciences, Engineering, and Medicine have suggested Chronic Disease Risk Reduction Intake limits from adult data extrapolation: < 1200 mg per day for ages 1–3 years, < 1500 mg per day for ages 4–8 years, < 1800 mg per day for ages 9–13 years, and < 2300 mg per day for ages 14–18 years.36 A pragmatic strategy for sodium intake reduction involves a diet with no added salt, cutting high-sodium processed foods, and educating families on how to read and understand food labels.
Healthy Diet: The DASH (Dietary Approaches to Stop HTN) diet was developed in the 1990s as a non-pharmacological method of lowering BP in adults.37 It includes fruits, vegetables, whole grains, lean meat, and low-fat dairy products. There is limited published data showing improves BP in pediatric populations on the DASH diet.38,39
Nutritional Counselling: Seek guidance from a registered dietitian for personalized dietary advice.
Physical Activity:
Regular Exercise: Minimum 60 minutes of moderate to vigorous physical activity, including walking, biking, swimming, or team sports most days of the week. A recent narrative review inferred the minimal impact of exercise on resting BP in adolescents with normal BP. However, it consistently lowered resting BP in adolescents with HTN.40
Limit sedentary lifestyle: Reducing screen time, including time spent on computers, tablets, and television, is important in non-pharmacological pediatric HTN management. A study on US adolescents found ⁓0.2 mmHg SBP increase for an hourly increment of sedentary activity.41
Weight Management:
Achieve and Maintain a Healthy Weight: Gradual weight loss through diet and exercise is crucial for overweight children. A balanced approach without rapid weight loss is crucial. A systematic review focusing on overweight/obese children found improvements in weight and BP (primarily diastolic BP by 1.69 mmHg) by incorporating lifestyle interventions.42
These non-pharmacological strategies may play a significant role in improving BP control and overall well-being in children with HTN. In reference to our case of primary HTN (10-year-old female), lifestyle modifications are recommended initially and may be effective in reducing BP to normotensive ranges – negating the need for pharmacological management. In contrast, the male, who had a history more suggestive of secondary HTN, will likely require more intensive management of his BP that includes both non-pharmacological and pharmacological management to achieve consistently normotensive BP measurements and reduce sequelae of poorly controlled BP.
Pharmacological management: In the pediatric population, medication becomes a consideration when lifestyle adjustments fail to reach BP targets, there’s a notable rise in BP accompanied by symptoms, a potentially treatable underlying cause is detected, or organ damage is evident. For uncomplicated HTN, both the AAP and HTN Canada guidelines advise starting with ACE inhibitors, ARBs, or long-acting CCBs.8,43 β-blockers are less preferred due to their side effects and specific cautionary notes regarding their use in individuals with asthma, diabetes, and those engaged in high-performance athletic activities. Refer to Table 8 for common pharmacological agents and their respective dosing guidelines for management of pedaitric hypertension.42,44-46
| Drug type | Drug | Starting dose | Maximum dose | Interval |
|---|---|---|---|---|
|
Angiotensin-converting enzyme inhibitors Common drug class side effects: cough, headache, dizziness, asthenia44 |
Benazepril45 | 0.2 mg/kg, up to 10 mg | 0.6 mg/kg, up to 40 mg | Daily |
| Captopril45 | 0.3-0.5 mg/kg | 6 mg/kg | Twice to three times daily | |
| Enalapril42 | 0.08 mg/kg/day | 0.6 mg/kg/day, up to 40 mg/day | Daily | |
| Fosinopril45 | 0.1-0.6 mg/kg | 40 mg | Daily | |
| Lisinopril45 | 0.08-0.6 mg/kg | 0.6 mg/kg, up to 40 mg | Daily | |
| Ramipril45 | 2.5 mg/m2 BSA | 6 mg/m2 BSA up to 10 mg | Daily | |
| Quinapril42 | 5-10 mg | 80 mg | Daily | |
|
Angiotensin receptor blockers Common drug class side effects: headache, dizziness44 |
Candesartan42 | 1-6 years: 0.2 mg/kg/day 6-17 years: <50 kg: 4-8 mg, >50 kg: 8-16 mg | 1-6 years: 0.4 mg/kg/day 6-17 years: 32 mg | Daily |
| Irbesartan45 | 75-150 mg | 300 mg | Daily | |
| Losartan45 | 0.7 mg/kg, up to 50 mg | 1.4 mg/kg, up to 100 mg | Daily | |
| Olmesartan42 | 20-35 kg: 10 mg, >35 kg: 20 mg | 20-35 kg: 20 mg, >35 kg: 40 mg | Daily | |
| Valsartan42 | <6 years: 5-10 mg/d, 6-17 years: 1.3 mg/kg/day, up to 40 mg | <6 years: 80 mg, 6-17 years: 2.7 mg/kg/day, up to 160 mg | Daily | |
|
Thiazide diuretics Common drug class side effects: hypokalemia, dizziness44 |
Chlorthalidone45 | 0.3 mg/kg | 2 mg/kg, up to 50 mg | Daily |
| Hydrochlorothiazide45 | 0.5-1 mg/kg | 3 mg/kg/day | Daily | |
|
Calcium channel blockers Common drug class side effects: peripheral edema, flushing, dizziness44 |
Amlodipine45 | 0.06-0.3 mg/kg | 5-10 mg | |
| Felodipine45 | 2.5 mg | 10 mg | Daily | |
| Nifedipine44 | 0.35-0.5 mg/kg | 3 mg/kg, up to 120 mg | Daily to twice daily | |
|
β-blockers Common drug class side effects: fatigue, diminished exercise ability, weight gain, worsening insulin sensitivity, onset of diabetes46 |
Labetalol45 | 2-3 mg/kg/day | 10-12 mg/kg/day, up to 1,200 mg/day | Twice daily |
| Atenolol45 | 1-3 mg/kg/day | 10-12 mg/kg/day, up to 1,200 mg/day | Twice daily | |
| Metoprolol45 | 0.5-1 mg/kg/day | 2 mg/kg/day | Daily to twice daily | |
| Carvedilol46 | 0.1 mg/kg per dose, up to 6.25 mg | 0.5 mg/kg per dose, up to 25 mg | Twice daily | |
|
α-blockers Common drug class side effects: postural hypotension with short-acting prazosin46 |
Prazosin45 | 0.05-0.1 mg/kg/day | 0.5 mg/kg/day | Three times daily |
| Doxazosin45 | 1 mg | 4 mg | Daily |
HTN: Hypertension, BSA: Body surface area, BP: Blood pressure
The treatment objectives for managing pediatric BP have evolved, driven by emerging trial data. They aim to establish consistent targets for adolescents in alignment with adult guidelines. According to the AAP guidelines, in children < 13 years, the goal is to achieve BP below the 90th percentile based on age and height. For adolescents (aged ≥13 years), aim for a BP <130/80 mmHg, reflecting the recommendations of the adult ACC/AHA guideline.8 Alternatively, the European pediatric guidelines adopt adult thresholds, suggesting a target BP < 140/90 mmHg for general HTN in adolescents (aged 16 years and older), and < 130/80 mmHg for those with diabetes mellitus.16 The SHIP-AHOY (Study of High Blood Pressure in Pediatrics: Adult HTN Onset in Youth) study defined BP categories for adolescents to evaluate their relationship with subclinical target organ injury (TOI) markers. BP was categorized into risk groups based on clinic and ABP readings: low-risk (below the 75th percentile), mid-risk (75th to 90th percentile), and high-risk (above the 90th percentile). These cutoffs outlined the likelihood of cardiovascular markers such as left ventricular hypertrophy, vascular stiffness, and altered cardiac function based on BP risk level. The study found a strong association between higher ABP levels, particularly SBP, and increased presence of multiple TOI markers, suggesting higher cardiovascular risk among adolescents with elevated BP.47
Current literature on pediatric patients with CKD has strong evidence of increased HTN prevalence with higher CKD stages. A prospective observational study by Schaefer et al. showed a 24.4% to 47.4% increase in the prevalence of HTN from CKD stage 3 to 5, and LVH prevalence was higher in the latter.48 Similarly, 48% of those being treated for HTN in the CKD study (n=585, children with CKD 1-16 years old) did not have adequate BP control.49 These studies affirm the increased HTN prevalence in the CKD population; however; in pediatric patients with CKD, current literature continues to have significant variability in suggested target BP cut-offs [Table 9].8,16,50-52
| Guideline | Cut-off/Target BP |
|---|---|
| European Society of Hypertension (ESH) 201616 | <75th percentile if no proteinuria <50th percentile if proteinuria |
| American Academy of Pediatrics (AAP) 20178 | <90th percentile (office BP) |
| Kidney Disease: Improving Global Outcomes (KDIGO) 202150 | <90th percentile (office BP) <50th percentile (ABPM) |
| National Institute for Health and Care Excellence (NICE) 202151 | <50th percentile if albumin-creatinine ratio >70 mg/mmol |
| KDIGO 202452 | 50th - 70th percentile (office BP) |
CKD: Chronic kidney disease, ABPM: Ambulatory blood pressure monitoring, BP: Blood pressure
Discrepancies in the current literature can confuse clinicians, underscoring the importance of further HTN trials and guideline standardization.
Pediatric HTN is a critical yet under-recognized condition with significant implications for both immediate and long-term health outcomes. Early identification through accurate BP measurement, comprehensive clinical evaluation, and appropriate diagnostic tools like ABPM are essential for timely intervention. Differentiating between primary and secondary HTN guides targeted management strategies, with lifestyle modifications forming the cornerstone. Pharmacological therapy and lifestyle modification with non-pharmacological measures should be considered. Recognizing the potential for target organ damage and increased cardiovascular risk in adulthood underscores the need for proactive, multidisciplinary care. By adopting a systematic approach to the diagnosis and management of pediatric HTN in the outpatient setting, healthcare providers can significantly improve long-term cardiovascular and renal health outcomes for affected children.
Conflicts of interest
There are no conflicts of interest.
References
- Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785-802.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global prevalence of hypertension in children: A systematic review and meta-analysis. JAMA Pediatr. 2019;173:1154-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of hypertension among children and adolescents in India: A systematic review and meta-analysis. Indian J Pediatr. 2021;88:1107-14.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood. Hypertension. 2020;75:948-55.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for hypertension in children and adolescents to prevent cardiovascular disease. Pediatrics. 2013;131:490-525.
- [CrossRef] [PubMed] [Google Scholar]
- Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: The cardiovascular risk in young finns study. J Pediatr. 2011;159:584-90.
- [CrossRef] [PubMed] [Google Scholar]
- Association of adolescent hypertension with future end-stage renal disease. JAMA Intern Med. 2019;179:517-23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904.
- [CrossRef] [PubMed] [Google Scholar]
- Definition and diagnosis of hypertension in children and adolescents. In: Flynn JT, Ingelfinger JR, eds. UpToDate. Waltham (MA): UpToDate, Inc.; 2024. Available from: https://www.uptodate.com
- [Google Scholar]
- Assessing blood pressure accuracy of an aneroid sphygmomanometer in a national survey environment. Am J Hypertens. 2011;24:322-7.
- [CrossRef] [PubMed] [Google Scholar]
- Oscillometric blood pressure: A review for clinicians. J Am Soc Hypertens. 2014;8:930-8.
- [CrossRef] [PubMed] [Google Scholar]
- 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269-324.
- [CrossRef] [PubMed] [Google Scholar]
- A standard protocol for blood pressure measurement in the newborn. Pediatrics. 1997;99:E10.
- [CrossRef] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, NIH. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. 2024. Available from: https://www.nhlbi.nih.gov/health-topics/fourth-report-on-diagnosis-evaluation-treatment-high-blood-pressure-in-children-and-adolescents [last accessed on 5 Jul 2024].
- American academy of pediatrics clinical practice guidelines for screening and management of high blood pressure in children and adolescents: What is new? Indian Pediatr. 2019;56:317-21.
- [CrossRef] [PubMed] [Google Scholar]
- 2016 European society of hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-920.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36:596-624.
- [CrossRef] [PubMed] [Google Scholar]
- Office blood pressure measurement alone often misclassifies treatment status in children with primary hypertension. Blood Press Monit. 2017;22:328-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ambulatory blood pressure monitoring in children and adolescents: 2022 update: A scientific statement from the american heart association. Hypertension. 2022;79:e114-2.
- [CrossRef] [PubMed] [Google Scholar]
- Survey of telemedicine by pediatric nephrologists during the COVID-19 pandemic. Kidney Int Rep. 2021;6:2316-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patients’ preference for ambulatory versus home blood pressure monitoring. J Hum Hypertens. 2014;28:224-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cost estimation of hypertension management based on home blood pressure monitoring alone or combined office and ambulatory blood pressure measurements. J Am Soc Hypertens. 2014;8:732-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of secondary hypertension in otherwise healthy youths with a new diagnosis of hypertension: A meta-analysis. J Pediatr. 2022;244:30-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Overview of monogenic or mendelian forms of hypertension. Front Pediatr. 2019;7:263.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- High blood pressure in children and adolescents: Current perspectives and strategies to improve future kidney and cardiovascular health. Kidney Int Rep. 2022;7:954-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk of target organ damage in children with primary ambulatory hypertension: A systematic review and meta-analysis. Hypertension. 2023;80:1183-96.
- [CrossRef] [PubMed] [Google Scholar]
- Adolescent blood pressure and the risk for early kidney damage in young adulthood. Hypertension. 2022;79:974-83.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term cardiovascular outcomes in children and adolescents with hypertension. JAMA Pediatr. 2024;178:688-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. 2022;386:1877-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cardiovascular risk factors in childhood and adulthood and cardiovascular disease in middle age. JAMA Netw Open. 2024;7:e2418148.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Blood pressure effects of sodium reduction. Circulation. 2021;143:1542-67.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sodium intake and blood pressure in children and adolescents: A systematic review and meta-analysis of experimental and observational studies. Int J Epidemiol. 2018;47:1796-810.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-9.
- [CrossRef] [PubMed] [Google Scholar]
- Oria M, Harrison M, Stallings VA, eds. Dietary reference intakes for sodium and potassium. Washington (DC): National Academies Press (US); 2019. (The National Academies Collection: Reports funded by National Institutes of Health). Available from: http://www.ncbi.nlm.nih.gov/books/NBK538102/
- Salt sensitivity and hypertension. J Hum Hypertens. 2021;35:184-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet DASH-sodium collaborative research group. N Engl J Med. 2001;344:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- Dietary approaches to stop hypertension dietary intervention improves blood pressure and vascular health in youth with elevated blood pressure. Hypertension. 2021;77:241-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Influence of dietary approaches to stop hypertension-type diet, known genetic variants and their interplay on blood pressure in early childhood: ABCD study. Hypertension. 2020;75:59-70.
- [CrossRef] [PubMed] [Google Scholar]
- Physical activity and blood pressure in adolescents. Pediatr Exerc Sci. 1994;6:361-80.
- [CrossRef] [Google Scholar]
- Dietary and lifestyle factors associated with blood pressure among U.S. adolescents. J Adolesc Health. 2007;40:166-72.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics. 2012;130:e1647-71.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertension Canada’s 2017 guidelines for the diagnosis, assessment, prevention, and treatment of pediatric hypertension. Can J Cardiol. 2017;33:577-85.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacologic treatment of chronic pediatric hypertension. Paediatr Drugs. 2005;7:27-40.
- [CrossRef] [PubMed] [Google Scholar]
- Narrative update of clinical trials with antihypertensive drugs in children and adolescents. Front Cardiovasc Med. 2022;9:1042190.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kaplan NM. Kaplan’s Clinical Hypertension. Available from: https://www.wolterskluwer.com/en/solutions/ovid/kaplans-clinical-HTN-762 [last accessed on 5 Jul 2024].
- SHIP-AHOY (Study of high blood pressure in pediatrics: Adult hypertension onset in youth) Hypertension. 2018;72:625-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cardiovascular phenotypes in children with CKD: The 4C study. Clin J Am Soc Nephrol. 2017;12:19-28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CKiD (CKD in children) prospective cohort study: A review of current findings. Am J Kidney Dis. 2012;60:1002-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1-S87.
- [CrossRef] [PubMed] [Google Scholar]
- Overview | Chronic kidney disease: assessment and management | Guidance | NICE. NICE; 2021. Available from: https://www.nice.org.uk/guidance/ng203 [last accessed on 10 Feb 2025].
- Executive summary of the KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease: Known knowns and known unknowns. Kidney Int. 2024;105:684-701.
- [CrossRef] [PubMed] [Google Scholar]







