Translate this page into:
Are Your Kidneys Ok? Detect Early to Protect Kidney Health
Corresponding author: Stefanos Roumeliotis, Second Department of Nephrology, American Hellenic Educational Progressive Association (AHEPA) University Hospital Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece. E-mail: st_roumeliotis@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vassalotti JA, Francis A, Dos Santos Jr ACS, Correa-Rotter R, Abdellatif D, Hsiao L, et al. Are Your Kidneys Ok? Detect Early to Protect Kidney Health. Indian J Nephrol. doi: 10.25259/IJN_252_2025
Introduction
Timely treatment is the primary strategy to protect kidney health, prevent kidney disease progression and related complications, reduce cardiovascular disease (CVD) risk, and prevent premature kidney-related and cardiovascular mortality.1-3 International population assessments show low awareness and detection of kidney disease and substantial gaps in treatment.2 People with kidney failure universally express the desire to have been diagnosed early to allow more time for educational, lifestyle, and pharmacologic interventions.4 Therefore, increasing knowledge and implementing sustainable solutions for early detection to protect kidney health are public health priorities.2,3
Epidemiology and complications of kidney disease
Chronic kidney disease (CKD) is highly prevalent, affecting 10% of the world’s population, or >700 million people.5 Almost 80% of the population with CKD reside in low-income countries (LICs) and lower middle-income countries (LMICs), with approximately 1/3 of the affected in China and India alone.5,6 CKD prevalence increased by 33% between 1990 and 2017.5 This increase is driven by population growth, aging, and the obesity epidemic, resulting in a higher prevalence of two major risk factors: type-2 diabetes (T2DM) and hypertension. In addition, risk factors beyond cardiometabolic conditions contribute to the rising CKD burden, including social deprivation, pregnancy-related acute kidney injury (AKI), preterm birth, and increasing environmental threats (infections, toxins, climate change, air pollution).5-7 These threats disproportionately affect people in LICs and LMICs.8
Undetected and untreated CKD is more likely to progress to kidney failure and cause premature morbidity and mortality. Globally, more people died in 2019 of CVD attributed to reduced kidney function (1.7 million people) than kidney disease alone (1.4 million).5 CKD is expected to rise to the 5th most common cause of years of life lost by 2040, surpassing type 2 diabetes, Alzheimer’s disease, and road injuries.9 The rising mortality of kidney disease is remarkable in contrast to other non-communicable diseases (NCDs) such as CVD, stroke, and respiratory disease, which are projected to experience a decline in mortality.8 Even in early-stage CKD, multi-system morbidity decreases quality of life. In particular, mild cognitive impairment is associated with early stage CKD and it is possible that early CKD detection and treatment could slow cognitive decline and reduce the risk of dementia.10 CKD in children has profound additional effects, threatening growth and cognitive development and with lifelong health and quality of life implications.11,12 It is anticipated that the number of people on kidney failure replacement therapy (KFRT) (dialysis and transplantation) will more than double to 5.4 million from 2010 to 2030.13,14 KFRT, especially hemodialysis, is unavailable or unaffordable to many in LICs and LMICs, contributing to millions of deaths annually. LICs and LMICs comprise 48% of the global population but account for only 7% of the treated kidney failure population.15
Who is at risk of kidney disease?
Testing people at high-risk for kidney disease (case-finding) limits potential harm and false-positives compared with general population screening, should only be considered in high-income countries (HICs). Limiting testing to those at increased risk of CKD would still capture a large proportion of the global population. Moreover, targeted case-finding in patients at high risk of CKD is not optimally performed, even in HICs. About 1/3 people worldwide have diabetes and/or hypertension. There is a bidirectional relationship between CVD and CKD, with each increasing the risk of the other. The American Heart Association and European Society of Cardiology call for testing those with CVD for CKD, as part of routine cardiovascular assessments.1,16
Other CKD risk factors include family history of kidney disease (e. g. APOL1-mediated kidney disease common in people of West African ancestry), prior AKI, pregnancy-related kidney disease (e.g. pre-eclampsia), malignancy, autoimmune disorders (systemic lupus erythematosus, vasculitis), individuals born with low birth weight or pre-term, obstructive uropathy, recurrent kidney stones, and congenital anomalies of the kidney and urinary tract (CAKUT), see Figure 1.3 The social determinants of health strongly affect CKD risk, both for individuals and at a country level. In LICs and LMICs, heat stress for agricultural workers is thought to cause CKD of unknown etiology, an increasingly recognized major global cause.17 In addition, envenomations, environmental toxins, traditional medicines, and infections (viral hepatitis B or C, HIV, and parasites) deserve consideration as risk groups, especially in endemic areas.18,19
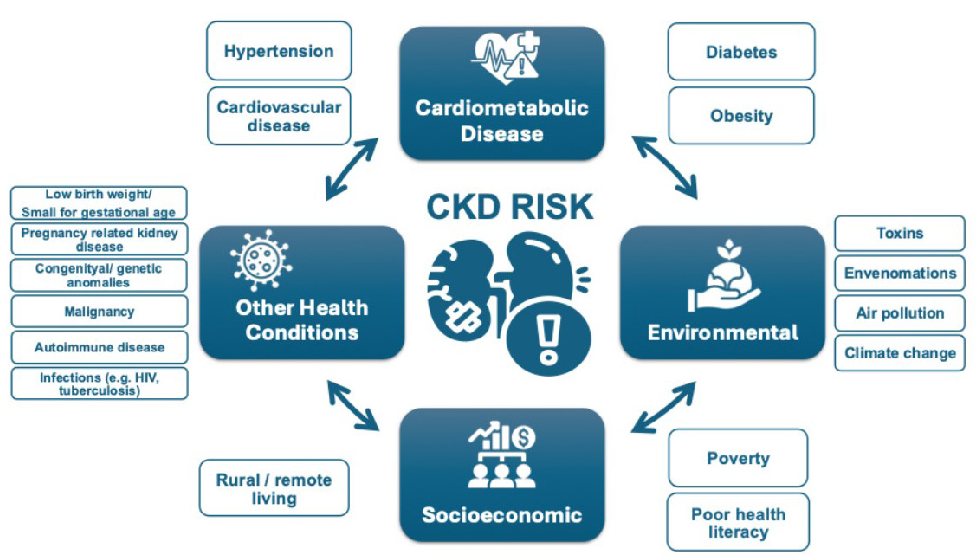
- Risk factors for CKD. CKD: Chronic kidney disease, HIV: Human immunodeficiency virus.
How can we check kidney health?
Conceptually, there are three levels of CKD prevention. Primary prevention reduces CKD incidence by treating risk factors; secondary prevention reduces progression and complications in patients; and tertiary prevention improves outcomes in those with kidney failure by improving management, such as improved vaccination or optimal dialysis delivery.20 Primary and secondary prevention strategies can utilize the 8 golden rules for kidney health promotion: healthy diet, adequate hydration, physical activity, blood pressure monitoring and control, glycemic monitoring and control, avoidance of nicotine, avoidance of regular use of non-steroidal anti-inflammatory drugs, and targeted testing for those with risk factors.21 Five of these are identical to ‘Life’s Essential 8’ rules for improving and maintaining cardiovascular health, which also include healthy weight, adequate sleep, and lipid management.22 Early detection focuses on secondary CKD prevention that involves protecting kidney health and reducing cardiovascular risk.
Are your kidneys okay?
Globally, early detection of CKD is rare, haphazard, and even less likely to occur in LICs or LMICs. Currently, only three countries have a national program to actively test for CKD in at-risk populations and a further 17 countries actively test the at-risk population during routine health encounters.23 Even in HICs, albuminuria is not assessed in >50% of people with T2DM and/or hypertension.24-26 Startlingly, in those with documented reduced kidney function, CKD diagnosis is often missing. A study in HICs showed the absence of CKD diagnosis among 62-96% of the population with laboratory evidence of CKD stage G3.27
We recommend that healthcare professionals perform the following tests for all risk groups to assess kidney health, see Figure 2:
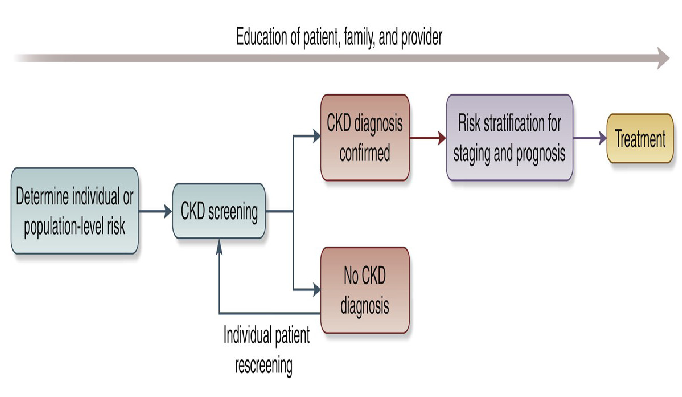
- Conceptual framework of a CKD testing, risk stratification, and treatment program, see reference.30 CKD: Chronic kidney disease.
a) Blood pressure measurements, as hypertension is the most prevalent risk factor for kidney disease worldwide.3,28,29
b) Body Mass Index (BMI), since obesity is epidemiologically associated with CKD risk indirectly through T2DM and hypertension, and as an independent risk factor. Visceral adiposity contributes to monocyte microinflammation and cardiometabolic kidney risk.3,28,29
c) Testing for diabetes with glycosylated hemoglobin or fasting blood sugar, or random glucose, is part of kidney health assessment, as T2DM is a common risk factor.3,28,29
d) Evaluating kidney function by using serum creatinine to estimate GFR (eGFR) is recommended in all settings.3 GFR should be estimated with a validated, race-free equation appropriate for the country or region and age group.3 In general, the eGFR < 60 mL/min/1.73m2 is the threshold for CKD in adults and children, although a threshold of < 90 mL/min/1.73m2 can be flagged as “low” in children and adolescents > 2 years.3 A limitation of creatinine-based eGFR is that creatinine is also a marker of nutrition and muscle mass. Therefore, states of malnutrition and frailty overestimate kidney function.3,30 Thus, eGFR using the combination of serum creatinine and cystatin C is generally more accurate than either biomarker alone in most clinical contexts. However, the feasibility of cystatin C use is mainly limited to HICs because of assay availability and cost relative to creatinine.3,30,31
e) Testing for kidney damage (albuminuria): In adults and children, a first morning sample is preferred for assessing albuminuria.3 In adults, quantitative urinary albumin-creatinine ratio (uACR) is the most sensitive test.3 Importantly, urinary albumin is still being standardized analytically, which should ultimately facilitate worldwide uACR standardization.32 In children, both protein-creatinine ratio (uPCR) and uACR should be tested to assess tubular proteinuria.3 Semiquantitative albuminuria testing allows for flexibility for point-of-care or home-based testing.33 Semiquantitative or qualitative screening tests should be positive in >85% of individuals with quantitative uACR 30 mg/g or more to be useful.34 In resource-constrained settings, urine protein dipstick testing may be used with a threshold of +2 proteinuria or greater to reduce false-positive results for repeat confirmatory testing.35
In specific populations, the following can be considered:
f) Testing for hematuria is notable as the forgotten risk factor in recent clinical practice guidelines. It is particularly important for those at risk for glomerular disease, particularly IgA nephropathy.36
g) Baseline imaging in groups with signs or symptoms of structural abnormalities (e.g. pain and hematuria) to evaluate for kidney masses, cysts, stones, hydronephrosis, or urinary retention is important. Antenatal ultrasound can detect hydronephrosis and other CAKUT.
h) With increasing availability of genetic testing, family cascade CKD testing is indicated for a known genetic risk of kidney disease.37
i) In those who have an occupational risk of developing kidney disease, kidney testing should be offered as part of occupational health programs.
j) Those who donate kidneys should be included in a post-donation surveillance program to assess kidney health over the long-term.38
Potential benefits of early detection
CKD screening fits with many of the World Health Organization’s Wilson-Jungner principles. Early-stage CKD is asymptomatic, and effective treatments, including lifestyle modification, interdisciplinary care, and pharmacologic interventions, have been established.2,3,30,35 WHO essential medicines that improve CKD outcomes should be widely available, including ACE inhibitors, angiotensin receptor blockers, statins, and sodium glucose co-transporter-2 inhibitors (SGLT2i).2,39 SGLT2i alone are estimated to decrease the risk of CKD progression by 37% in people with and without diabetes.40 For a 50-year old person with albuminuria and non-diabetic CKD, this could extend their future period of healthy kidney function from 9.6 years to 17 years.41 These essential medicines reduce progression to more advanced CKD stages and limit cardiovascular hospitalization to provide short-term cost-effectiveness, especially for LICs. Where available and affordable, the range of new paradigm-shifting medication to slow CKD progression also includes glucagon-like peptide-1 receptor antagonists, non-steroidal mineralocorticoid receptor antagonists, endothelin receptor antagonists, and specific disease-modifying drugs (e.g., complement-inhibitors) that herald an exciting new era for nephrology.
Considering the significant healthcare costs associated with CKD, particularly hospitalization and kidney failure, effective preventive measures offer clear economic benefits for both high- and LICs. CKD confers enormous costs to the individual, their families, healthcare systems, and governments worldwide. In the United States, CKD costs Medicare over US$ 85 billion annually.13 In many high- and MICs, 2-4% of the health budget is spent on kidney failure care alone. In Europe, healthcare costs associated with CKD are higher than those associated with cancer or diabetes.42 Reducing the kidney care burden worldwide will also have profound environmental effects, saving water and plastic waste, especially that associated with dialysis.43 CKD costs are frequently catastrophic for individuals in LICs and LMICs, where the individual largely bears the burden of payment. Only 13% and 19% of LICs and LMICs, respectively, cover the KFRT costs for adults.15 CKD causes 188 million people in LICs and LMICs annually to be faced with catastrophic healthcare expenditures.44
The most widely cited and studied incremental cost effectiveness ratio (ICER) threshold to assess screening is US$ <50,000 per quality-adjusted life year (QALY).45 If the CKD prevalence is high, a population-wide screening strategy should be considered in HICs.33,46 For example, in the United States, a recent Markov simulation model of population-wide CKD screening, which included appropriate SGLT2i treatment added to standard of care ACE inhibitors or angiotensin receptor blockers for adults age 35 to 75 years old with albuminuria, concluded that screening would be cost-effective.46 In addition, an analysis of a home-based general population semiquantitative albuminuria screening in Holland was also found to be cost-effective.33 Case finding to detect CKD in higher risk groups rather than mass or general population screening will reduce costs and other harms, whilst increasing the screening tests’ true positive rate.3,35,45 An alternate ICER threshold proposed by WHO is <1-3 times the ratio of the gross domestic product per capita income per QALY can be used to assess case finding approaches in LIC and LMIC.45 The recommended tests for detecting kidney disease are low-cost and minimally invasive, facilitating their administration across diverse settings. Basic eGFR and urinary ACR tests are widely available, and urine dipstick testing, in places with unavailable and unaffordable proteinuria, will drastically reduce testing costs.31
If coupled with effective intervention, early identification affected individuals will benefit the individual, the health care system, governments, and the economy.44 Health and quality of life benefits for the individual would lead to improved productivity, especially in the young with more working years ahead, and to developmental/educational improvements in children and young adults. Individuals would face less catastrophic health expenses, governments and healthcare systems will save money not only on CKD care, but also on CVD costs, and economies will benefit from more worker participation. This is especially crucial for LICs, where the greatest CKD burden exists and is cruelly coupled with the lowest ability for governments and individuals to afford kidney care.
Challenges and solutions for implementation
Structural barriers to widespread identification and treatment of people with CKD include cost, reliability of testing, and lack of health information systems to track burden. These are underpinned by a lack of relevant government and healthcare policy, low healthcare professional knowledge and implementation, poor general population perceived kidney disease risk, and low patient awareness. Solutions for implementing effective interventions include tying identification to existing screening programs, educating the public and primary care professionals, and leveraging non-governmental organization (NGO) joint advocacy programs to focus health policy agendas on kidney disease. Any solutions must balance the potential benefits and harms of screening and case-finding programs. Ethical implications for consideration include the availability of resources (such as health care workers and medicines), the affordability of testing and treatment, false positives or negatives, and anxiety for patients and their families.47
Screening and case-finding programs require workforce capacity, health information systems, reliable testing equipment, and equitable access to medical care, medicines, vaccines, and medical technologies. Primary care is at the front lines to protect kidney health, particularly in LICs and LMICs. The tiny nephrology workforce, with a median global prevalence of 11.8 nephrologists per million population and an 80-fold difference between LICs and HICs, is inadequate to detect and manage the vast CKD majority.23 As for other chronic diseases, primary care clinicians and other frontline health workers are foundational to early detection.48 Testing must be affordable, simple, and practical, with point-of-care creatinine testing and urine dipsticks being useful in resource-limited settings.31 Educational efforts directed at primary care clinicians are key to integrating CKD detection into routine care, despite constrained time and resources.49-51 Automated clinical decision support could leverage electronic health records to identify people with CKD or at high-risk and recommend appropriate actions to clinicians [Figure 2].
Currently, few countries have CKD registries, limiting our ability to highlight the disease burden to governments. Knowledge of CKD burden assists in prioritizing kidney health needs, which should then progressively expand to encompass the full kidney care spectrum.52 A global survey revealed only a quarter of the countries (41/162) had a nation-specific CKD strategy and fewer than a third (48/162) recognized CKD as a public health priority.23 WHO’s recognition of CKD as a major driver of NCD mortality would be impactful in increasing awareness, improving local surveillance and monitoring to implement clinical practice guidelines, and improving resource allocation.2
Programs for the early CKD detection will require extensive coordination and engagement of stakeholders, including governments, health systems, and insurers. International and national kidney organizations, such as the International Society of Nephrology (ISN), already advocate to the WHO and individual governments for the prioritization of kidney disease. We must continue this work, collaborating to streamline early detection program planning and implementation. Connection to existing community interventions (e.g., CVD prevention) in LMICs and HICs can decrease cost and maximize efficiencies by integrating into existing programs. Such programs will need to be adapted to the local context and can be held in a variety of settings, such as individual healthcare practices, hospitals, as well as regional or national healthcare facilities, or as outreaches in rural communities. Depending on local regulations and resources, screening and case-finding can also take place outside medical settings such as town halls, churches, or markets. Volunteers in the community can also assist with community-based screening and case-finding efforts.
In conjunction with reorienting the clinical practice of health care professionals to a greater focus on timely CKD detection, we must focus on general population perceived risk education and health promotion activities, as well as education programs aimed at patient awareness and empowerment. General population awareness of kidney disease is poor, with 9/10 people with CKD unaware they are affected.53 Kidney disease awareness is missing in mainstream conversation, with a lay press analysis showing kidney disease being 11 times under-represented in discussed compared to the actual cause of death.54 A number of national and international organizations have developed public-facing quizzes on the kidney disease risk, supported by a regional study that showed socially vulnerable patients with hypertension do not understand their kidney risks.21,55-57 Online and direct education for healthcare professionals can improve consumer health literacy. Patient activation, engagement, and shared decision-making are downstream impacts of awareness. Awareness education is nuanced for CKD, including detection and risk stratification to inform and empower rather than frighten regarding the timing and extent of interventions [Box 1].4,27,57 Getting the balance right will optimize self-efficacy and patient, family, and caregiver engagement.
A Call to Action
We call on all healthcare professionals to check the kidney health of their patients at risk of kidney disease. In tandem, we must work with public health organizations to improve the general population’s perceived risk of kidney disease and empower people at risk to seek kidney health checks. To ensure this change can be delivered, we must work with healthcare systems, governments, and the WHO to prioritize kidney disease and create effective and efficient early detection programs for kidney disease. Only then will the paradigm-shifting benefits of lifestyle change, and pharmacologic treatments translate to better kidney and overall health for people all around the world.
Conflicts of interest
There are no conflicts of interest.
References
- A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: A scientific statement from the american heart association. Circulation. 2023;148:1636-64.
- [CrossRef] [PubMed] [Google Scholar]
- Mind the gap in kidney care: Translating what we know into what we do. Kidney Int. 2024;105:406-17.
- [CrossRef] [PubMed] [Google Scholar]
- KDIGO 2024 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105:S117-34.
- [CrossRef] [PubMed] [Google Scholar]
- Patient needs and priorities for patient navigator programmes in chronic kidney disease: A workshop report. BMJ Open. 2020;10:e040617.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709-33.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Representation of low- and middle-income countries in ckd drug trials: A systematic review. Am J Kidney Dis. 2025;85:55-66.
- [CrossRef] [PubMed] [Google Scholar]
- Kidney health for all: Preparedness for the unexpected in supporting the vulnerable. Kidney Int. 2023;103:436-43.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic kidney disease and the global public health agenda: An international consensus. Nat Rev Nephrol. 2024;20:473-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet. 2018;392:2052-90.
- [Google Scholar]
- Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020;16:452-69.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neurocognitive and educational outcomes in children and adolescents with CKD: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2018;13:387-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of life of children and adolescents with chronic kidney disease: A cross-sectional study. Arch Dis Child. 2019;104:134-40.
- [CrossRef] [PubMed] [Google Scholar]
- 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2023.
- Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet. 2015;385:1975-82.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of global kidney health care status. JAMA. 2017;317:1864-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease as cardiovascular risk factor in routine clinical practice: A position statement by the Council of the European Renal Association. Nephrol Dial Transplant. 2023;38:527-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic Kidney Disease of Unknown Cause in Agricultural Communities. N Engl J Med. 2019;380:1843-52.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of access to kidney care for children in low-resource settings. Nat Rev Nephrol. 2021;17:33-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. 2016;31:868-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comprehensive public health strategies for preventing the development, progression, and complications of CKD: Report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53:522-35.
- [CrossRef] [PubMed] [Google Scholar]
- World Kidney Day 2025. Available from: https://www.worldkidneyday.org/about-kidney-health/ [last accessed on 15 Apr 2025].
- Life’s essential 8: Updating and enhancing the American heart association’s construct of cardiovascular health: A presidential advisory from the American heart association. Circulation.. 2022;146:e18-e43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health. 2024;12:e382-95.
- [CrossRef] [PubMed] [Google Scholar]
- Fulfillment and validity of the kidney health evaluation measure for people with diabetes. Mayo Clin Proc Innov Qual Outcomes. 2023;7:382-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- chronic kidney disease testing among at-risk adults in the U.S remains low: Real-world evidence from a national laboratory database. Diabetes Care. 2021;44:2025-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 u.S health care organizations. Diabetes Care. 2021;44:2000-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patient and clinician perspectives: to create a better future for chronic kidney disease, we need to talk about our kidneys. Adv Ther. 2024;41:1318-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Screening, identifying, and treating chronic kidney disease: Why, who, when, how, and what? BMC Nephrol. 2024;25:34.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CKD screening for better kidney health: Why? Who? How? When? Nephrol Dial Transplant. 2024;39:1537-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The case for early identification and intervention of chronic kidney disease: Conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2021;99:34-47.
- [CrossRef] [PubMed] [Google Scholar]
- Availability and affordability of kidney health laboratory tests around the globe. Am J Nephrol. 2020;51:959-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Urine Albumin measurements in clinical diagnostics. Clin Chem. 2024;70:382-91.
- [CrossRef] [PubMed] [Google Scholar]
- Screening for chronic kidney disease: Change of perspective and novel developments. Curr Opin Nephrol Hypertens. 2024;33:583-92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2023;69:808-6.
- [CrossRef] [PubMed] [Google Scholar]
- Early detection of CKD: Implications for low-income, middle-income, and high-income countries. J Am Soc Nephrol. 2020;31:1931-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Haematuria: The forgotten CKD factor? Nephrol Dial Transplant. 2012;27:28-34.
- [CrossRef] [PubMed] [Google Scholar]
- Advancing genetic testing in kidney diseases: Report from a national kidney foundation working group. Am J Kidney Dis. 2024;84:751-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Barriers to accessing essential medicines for kidney disease in low- and lower middle-income countries
- Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788-801.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Estimated lifetime benefit of combined RAAS and SGLT2 inhibitor therapy in patients with albuminuric CKD without diabetes. Clin J Am Soc Nephrol. 2022;17:1754-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat Rev Nephrol. 2017;13:393-409.
- [CrossRef] [PubMed] [Google Scholar]
- Combining patient care and environmental protection: A pilot program recycling polyvinyl chloride from automated peritoneal dialysis waste. Kidney Int Rep. 2024;9:1908-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Economic burden of chronic ill health and injuries for households in low- and middle-income countries. In: Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, eds. Disease Control Priorities: Improving Health and Reducing Poverty. Washington (DC): The International Bank for Reconstruction and Development / The World Bank © 2018 International Bank for Reconstruction and Development / The World Bank.; 2017.
- [Google Scholar]
- Cost-effectiveness of screening for chronic kidney disease in the general adult population: A systematic review. Clin Kidney J. 2023;17:sfad137.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Population-wide screening for chronic kidney disease : A cost-effectiveness analysis. Ann Intern Med. 2023;176:788-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ethical issues related to early screening programs in low resource settings. Kidney Int Rep. 2024;9:2315-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Primary care detection of chronic kidney disease in adults with type-2 diabetes: The ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PLoS One. 2014;9:e110535.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129:153-62.
- [CrossRef] [PubMed] [Google Scholar]
- CKD for primary care practitioners: can we cut to the chase without too many shortcuts? Am J Kidney Dis. 2016;67:826-9.
- [CrossRef] [PubMed] [Google Scholar]
- Integrating CKD into us primary care: bridging the knowledge and implementation gaps. Kidney Int Rep. 2022;7:389-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Priority setting as an ethical imperative in managing global dialysis access and improving kidney care. Semin Nephrol. 2021;41:230-41.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic Kidney Disease in the United States 2023 Available from: https://www.cdc.gov/kidneydisease/publications-resources/CKD-national-facts.html [last accessed on 15 Apr 2025].
- Does the news reflect what we die from? 2019. Available from: https://ourworldindata.org/does-the-news-reflect-what-we-die-from [last accessed on 8 Sep 2022].
- Perceived susceptibility to chronic kidney disease among high-risk patients seen in primary care practices. J Gen Intern Med. 2009;24:1123-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Foundation NK. Kidney Quiz 2024. Available from: https://www.kidney.org/kidney-quiz/ [last accessed on 15 Apr 2025].
- Usability testing of the kidney score platform to enhance communication about kidney disease in primary care settings: Qualitative think-aloud study. JMIR Form Res. 2022;6:e40001.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






