Translate this page into:
Squamous Cell Carcinoma of Skin after 20 Years of Renal Transplantation
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Solid organ transplant recipients are at high risk of developing malignancies due to the prolonged use of immunosuppressant drugs. Squamous cell carcinoma of skin can occur in these patients even after decades of organ transplant. A 45-year-old male underwent renal transplant for end-stage renal disease 23 years ago and was on immunosuppressive drugs since then. The patient was on regular follow-up. Three years back, he developed squamous cell carcinoma of both forearms and hands, which was treated with radiation therapy using 8 MeV electrons, by parallel opposed fields to a dose of 60 Gy/30 fractions. Complete response to treatment was achieved at 3 months posttreatment. The patient is currently on follow-up and asymptomatic for skin lesions. Hence, these patients require longer follow-up, active surveillance, and screening for early diagnosis and prompt treatment of the premalignant and malignant conditions.
Keywords
Immunosuppressant
malignancy
renal transplantation
squamous cell carcinoma
Introduction
With the advent of highly efficacious immunosuppressant drugs, the survival of patients with organ transplant has improved dramatically. Unfortunately, it has also increased the risk of developing malignancies, which increases with the increasing duration of immunosuppression.[1] Although the risk is well known, it is imprecisely quantified. We report a case of a renal transplant patient developing squamous cell carcinoma, 20 years after renal transplantation. The purpose is to draw attention toward the need of long follow-up, active surveillance, and the measures required for early diagnosis and prompt treatment.
Case Report
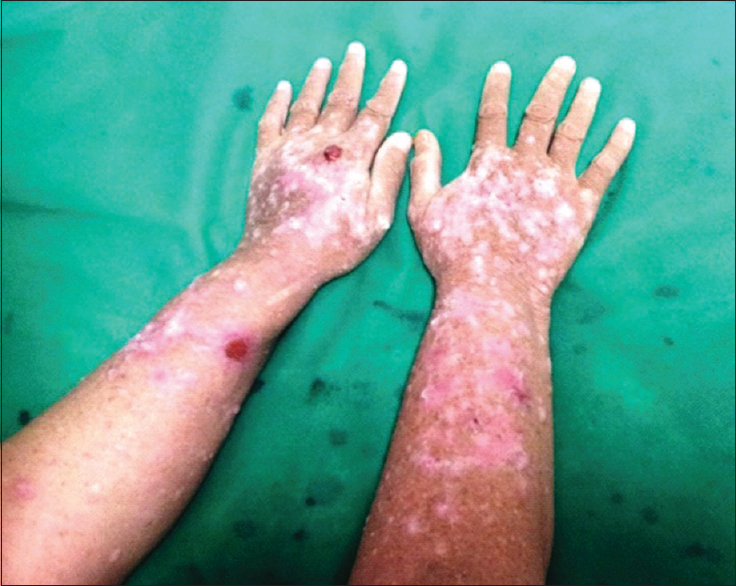
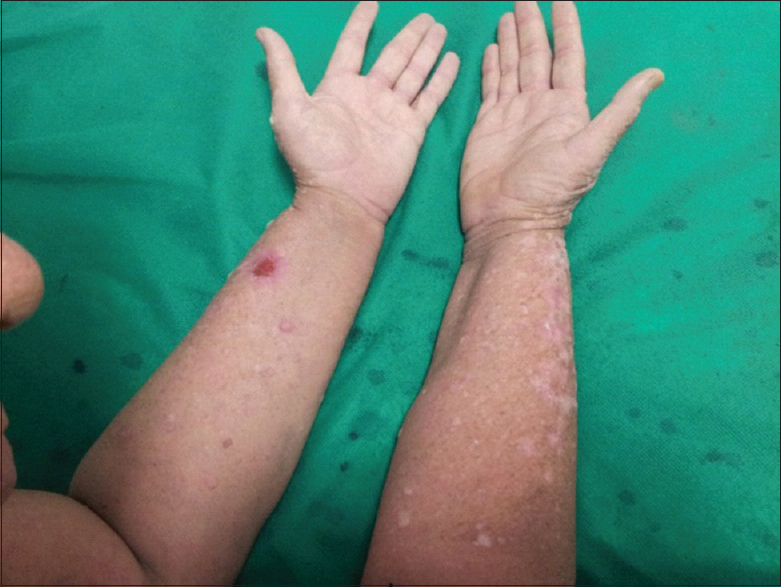
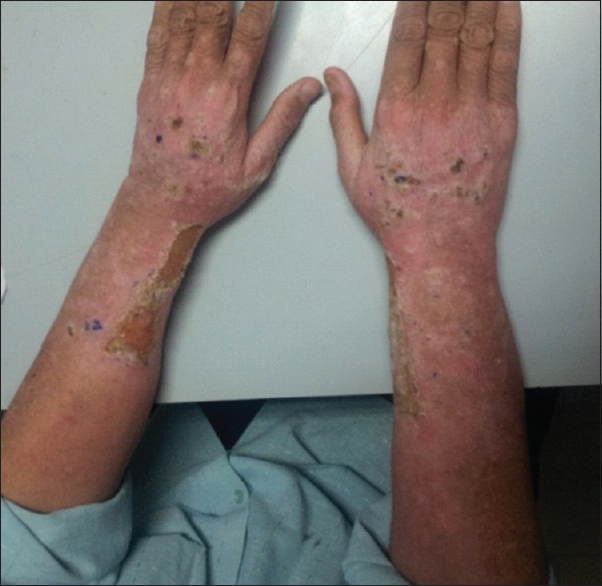
A 45-year-old male underwent renal transplant for end-stage renal disease due to glomerulonephritis, 23 years ago, and was started on immunosuppressive drugs (cyclosporine and azathioprine). He has history of excessive sun exposure, because of his profession. The patient was alright and was on regular follow-up. Three years back, he developed itchy, irregular, papular lesions over both forearms and hands. The biopsy was suggestive of actinic keratosis and was treated with chemical peeling. Lesions rapidly progressed to cover both forearms and hands, on flexor and extensor aspect [Figures 1–3]. A repeat biopsy confirmed squamous cell carcinoma [Figure 4]. The patient was prescribed topical 5-fluorouracil but with no relief. Finally, he was referred for radiotherapy to our center. Electron beam therapy with 8 MeV electrons, with parallel opposed anteroposterior portals, was planned for a dose of 60 Gy in 30 fractions, from September 24, 2012, to November 5, 2012. The dose of immunosuppressants was reduced. The patient tolerated the treatment well with only Grade III skin reactions. Complete response to treatment was achieved at 3 months after the electron therapy [Figures 5 and 6]. Currently, the patient is on regular follow-up and asymptomatic for the skin lesions. The graft function continues to remain stable. The Institutional Ethics Committee was informed, and due permission was taken for reporting this case as a case report.
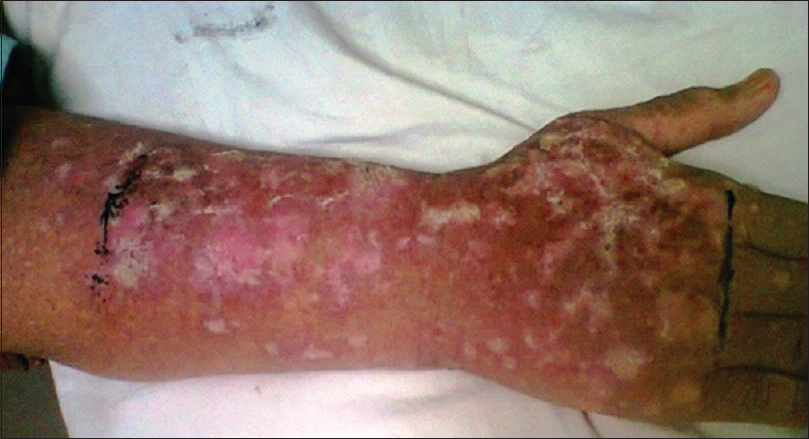
- Lesions on arm and forearm
- Lesions of forearm
- Lesions on flexor aspect of both forearm and hands
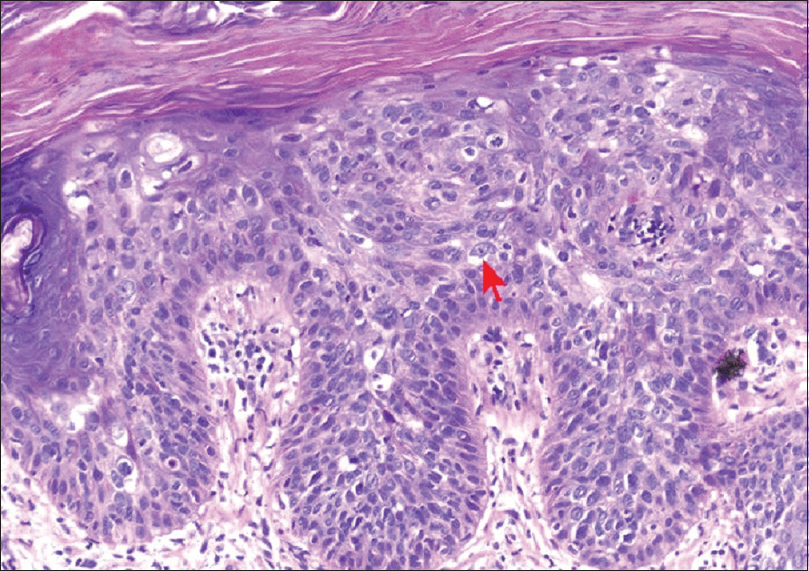
- Histopathology slide showing intraepidermal atypical squamous cell
- Response to radiation therapy after 1 month of treatment completion
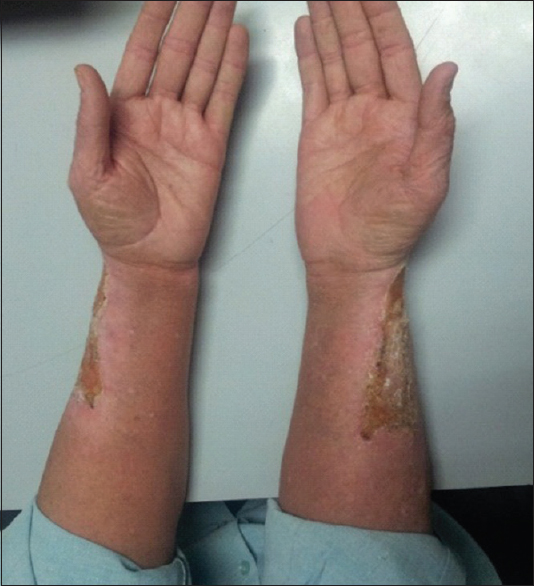
- Response to radiation therapy after 3 months of treatment completion
Discussion
Solid-organ transplant recipients are three to five times more vulnerable to develop malignancy.[2] The cause is attributed to the immunosuppressive treatment.[3] These drugs impair immune reactions against viral infections, most common being human papillomavirus (HPV).[3] In addition, inactivation of p53 gene occurs due to exposure of the skin to ultraviolet radiation which causes DNA mutations. Malignant proliferation is a result of the failure to repair such mutations.[4]
The duration and intensity of immunosuppression, sunlight exposure, and age at transplantation are powerful risk factors for the development of skin malignancy. Others are pale skin, viral infections (HPV), precancerous lesions such as warts and cigarette smoking.[5] The most frequent types of tumors are posttransplant lymphoproliferative disorders (PTLD) and squamous cell carcinoma of lip and skin. Squamous cell carcinoma accounts for 40%–53% of all malignancies in these patients.[6] Less frequent types are basal cell carcinoma, melanoma, Kaposi's sarcoma, and Merkel cell carcinoma.[6] The incidence of PTLD is highest within first 3 years of transplant, whereas squamous cell carcinoma can occur as late as 20 years posttransplant. The incidence of malignancy increases gradually after 10 years of the transplant, and it becomes as high as 13.8-fold higher in these patients as compared to the normal population and the cumulative frequency increases with the increase in the duration of the follow-up. Prevention and screening may play an important role in reducing the morbidity and mortality of malignancy in these patients. Preventive measures include avoidance of sun exposure, monitoring the intensity of immunosuppressive drugs, and checking viral and fungal infections.[7] Secondary prevention aims at early detection and prompt treatment. These patients should be examined thoroughly on a routine basis both by self and a physician to detect precancerous lesions and early cancers.[7]
Precancerous lesions could be treated with cryosurgery, chemopeeling, dermabrasion, and carbon dioxide laser. Treatment options for squamous cell carcinoma are curettage and desiccation, Mohs’ micrographic technique, and cryotherapy. For extensive involvement of skin cancers, intensity of the immunosuppressive drugs should be reduced and adequate radiation therapy should be delivered. Radiation therapy is preferred in patients where the lesion is not amenable to surgery or the patient is not fit for surgery. It can also be used as an adjuvant treatment following surgery.[8] Radiation can be delivered by 6 MV photons or electrons of the range 6–10 MeV. Electrons are preferred over photons, if available, as the dose to the skin is better with quick fall-off is surrounding and underlying tissues.
Conclusion
Transplant recipients require long and thorough follow-up examinations. They are at high risk of developing malignancy, viral infections such as hepatitis (hepatitis B virus, hepatitis C virus), and disseminated fungal infections, for example, Candida and Aspergillus. Although the viral and fungal infections occur during the initial few years of posttransplant, malignancy can occur as long as 20 years after the transplant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- (2014, March 25) Skin Cancers in Transplant Patients. Skin Cancer Foundation. Retrieved from http://www.skincancer.org/prevention/are-you-at-risk/transplant
- [Google Scholar]
- Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390-5.
- [Google Scholar]
- Malignancy after renal transplantation: The role of immunosuppression. Nature Reviews Nephrology. 2010;6(9):511-9.
- [Google Scholar]
- Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177-86.
- [Google Scholar]
- Cancer in the transplant recipient. Cold Spring Harb Perspect Med 2013:3. pii: a015677
- [Google Scholar]







