Translate this page into:
Efficacy and Safety of Cyclosporine versus Tacrolimus in Steroid and Cyclophosphamide Resistant Nephrotic Syndrome: A Prospective Study
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Calcineurin inhibitors (CNIs) are the preferred drugs for treatment of childhood steroid-resistant nephrotic syndrome (SRNS) who are also resistant to cyclophosphamide (CYC). Although few studies have shown a benefit of one over the other, efficacy and safety of either CNIs (tacrolimus [TAC] or cyclosporine [CSA]) in this special population remained to be assessed in long-term studies. Forty-five children with SRNS who were also resistant to CYC (CYC-SRNS) from January 2006 to June 2011, were included in the study. Patients were treated with CNI either TAC or CSA based on 1:1 allocations and were prospectively observed. Patients who were nonresponsive to CNIs had been treated with mycophenolate mofetil. Outcomes were measured in terms of remission of NS, adverse effects of drugs, and progression of disease. After 6 months of treatment, 16/23 (69.5%) patients on CSA achieved remission and 18/22 (81.8%) on TAC achieved remission (P = 0.3). The side effects hypertrichosis, and gum hyperplasia were significantly less in TAC group as compared to CSA group (P < 0.001). The 1-, 2-, 3-, 4-, and 5-year estimated renal survival (doubling of serum creatinine as event) in CSA group was 96%, 91%, 85%, 54%, and 33% and in TAC group was 96%, 95%, 90%, 89%, and 79%, respectively (P = 0.02). Although TAC and CSA are equally efficacious, TAC has significantly less side effects. The long-term outcome of renal function was significantly better in patients who were treated with TAC as compared to CSA.
Keywords
Cyclosporine
cyclophosphamide resistance
nephrotic syndrome
steroid resistance
tacrolimus
Introduction
Idiopathic nephrotic syndrome (INS) is one of the most common glomerular diseases in children. Approximately 80%–90% of the patients are steroid responsive, 40%–60% of them experience frequently relapsing or steroid-dependent course despite initial clinical response, and about 10%–20% of children treated for new onset NS remain resistant to steroid treatment.[1] Incidence of steroid resistance NS (SRNS) is increasingly observed in South Asians.[2] The treatment of SRNS with multiple courses of cytotoxic agents, and calcineurin inhibitors (CNIs) remains a challenging clinical scenario.[23]
Cyclophosphamide (CYC) is reported to induce remission in only 25%–30% of these patients.[1] Patients who are resistant to CYC and steroid (CYC-SRNS patients) have limited treatment options such as CNIs, mycophenolate mofetil (MMF), and rituximab.[4] Managing these patients is challenging as these patients are at risk of complications of unremitting NS, progression to end-stage renal disease (ESRD) at one extreme, and adverse effect of immunosuppressions on the other.[23] Twelve percent of pediatric ESRD patients are due to focal segmental glomerulosclerosis (FSGS), making it one of the most prevalent acquired kidney diseases.[5] Several different therapies have been attempted, all with variable degrees of success. Improving quality of life and preventing disease progression, even partial, are associated with the preservation of renal function and favorable long-term outcome.[4]
Currently, there is a paucity of data on efficacy of CNIs (Cyclosporine [CSA] and Tacrolimus [TAC]) in CYC-SRNS patients. Both CNIs vary in the immunosuppressant potency and side effects profile.[6] Published data have heterogeneous population with steroid dependent along with SRNS patients with various histopathological subtypes.[789] Except for sparse case reports[8910] and a randomized controlled trial (RCT)[11] with only 17 out of 41 SRNS patients being CYC-SRNS patients, there are no studies on efficacy of CNI in this special population. Hence, this study was done to assess the efficacy and safety of CSA versus TAC in CYC-SRNS patients.
Materials and Methods
CYC-SRNS patients with onset of disease between age 2 and 16 years and biopsy-proven minimal change disease (MCD) or FSGS were included in the study. Patients with estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, family history of NS, and patients with any evidence of secondary causes for NS such as lupus nephritis, and other glomerular diseases like membranous nephropathy and IgA nephropathy were excluded from the study. Patients with diabetes, epilepsy, prior treatment other than CYC (CNI, MMF, and rituximab) were also excluded from the study. Patients enrolled in this study were negative for hepatitis B, hepatitis C, and human immunodeficiency virus infection and had normal serum complement (C3 and C4) levels.
Study definitions
Steroid resistance was defined as nonresponsiveness to 60 mg/m2/d prednisone therapy for 4 weeks. Primary steroid resistance was defined as failure to achieve remission in 4 weeks of therapy. Patients who did not achieve remission after 4 weeks of treatment, but remitted once previously, were defined as secondary steroid resistant. CYC resistance was defined by failure to induce remission after 12 weeks of treatment. Complete remission (CR) was defined by either urinary protein to creatinine ratio (uPCR) <200 mg/g (<20 mg/mmol) or <1+ of protein on urine dipstick for 3 consecutive days. Partial remission was defined as proteinuria reduction of >50% or greater from the presenting value and absolute uPCR between 200 and 2000 mg/g. CNI resistance at 6th month was defined by the persistence of urine protein excretion >50% from baseline or persistent excretion uPCR >2000 mg/g after completion of 6 months of therapy.
CNI resistance at 12th month was defined as the inability to induce a CR. Relapse after CNI withdrawal was defined by recurrence of uPCR ≥2000 mg/g (≥200 mg/mmol) or ≥3+ protein on urine dipstick for 3 consecutive days. Hypertension was defined as a systolic blood pressure or a diastolic blood pressure greater than the 95th percentile for age and sex measured on at least three separate occasions. eGFR was calculated by Schwartz formula.[12]
Study design and protocol
The study was conducted as shown in Figure 1 as an investigator-initiated prospective study. Forty-five CYC-SRNS patients (From January 2006 to June 2011) were categorized into two groups based on 1:1 distribution to receive either CSA or TAC. CSA (Neoral, Novartis, Mumbai, Maharashtra India) was used at a dose of 5 mg/kg/day and TAC (Tacsant, Novartis, Mumbai, Maharashtra, India) at a dose of 0.1 mg/kg/day as per our experience in the previous study.[13] CSA and TAC dose was titrated to maintain a trough level of 120–180 ng/ml and 6–10 ng/ml, respectively. Drug level (CSA or TAC) was done at 2nd week and then at 2nd month. Further drug levels were only done if worsening of renal function or resistance is noted. The mean trough TAC level at 2nd week was 7.67 ± 2.2 ng/ml (95% confidence interval [CI]: 6.66–8.62, min 3.1–max 10.9) and at 2nd month was 7.60 ± 1.38 (95% CI: 6.98–8.21, min 4.8–max 10.4). CSA dose at 2nd week was 160.82 ± 41.4 ng/ml (95% CI: 142.91–178.73, min 82–max 235) and 2nd month was 160.08 ± 26.5 (95% CI: 148.59–171.57, min 105–max 208).
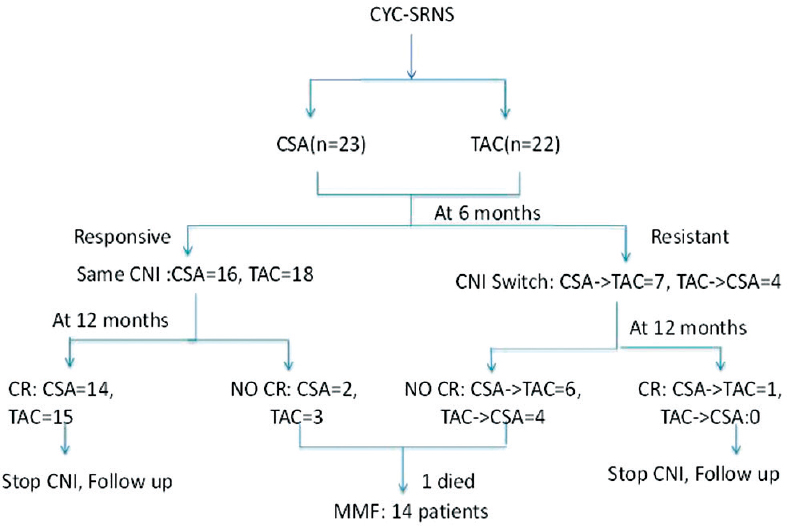
- Flow chart of the study
All patients received prednisolone, enalapril, calcium, and Vitamin D supplements in both groups. Prednisolone was used at 0.5 mg/kg/day. Enalapril was used at 0.2 mg/kg/day. If needed for hypertension control, then dose increased to 0.3 mg/kg/day or a maximum of 20 mg per day. Patients still remaining hypertensive received nifedipine 10–30 mg thrice a day.
At the end of 6 months, patients who did not achieve either partial or CR were declared as nonresponsive to 1st CNI, and these patients were switched over to another group of CNI from CSA to TAC or vice versa. The basis of switch to another CNI was based on the evidence that maximum patients respond to the CNI by 6 months.[8] The same CNI was continued in all responsive patients with either CR or PR for 12 months, and steroid was tapered at 25% per month and stopped. Relapse following CNI withdrawal was retreated with prednisone 2 mg/kg/d or 60 mg/m2/d for 4 weeks followed by 1.5 mg/kg or 40 mg/m2 per dose alternate day for 4 weeks, if responsive, then steroid tapered by 25% per month and stopped over 6 months. If steroid resistant, then patients were retreated with the CNI to which they responded previously.
The primary end point of the study was remission (either partial or complete) at the end of 6 months and 12 months of 1st CNI. The secondary end points were response to 2nd CNI at 6 months, decline in eGFR, and determination of renal survival (by doubling of baseline serum creatinine), adverse effects of CNI, and relapse of NS on follow-up.
Clinical and biochemical monitoring
Patients had their first follow-up at the end of 2nd week and then every 2 months. At follow-up, every patient had a subjective and objective clinical assessment for adverse effects. Blood tests were done for hemogram, serum creatinine, lipids, and transaminase. Urine was assessed for spot protein and creatinine ratio. Schwartz formula was used to calculate eGFR for patients. Patients were followed up for 74 months in CSA group and 72 months in TAC group and each patient in either group had minimum follow-up of 24 months.
Informed consent was obtained from a parent or guardian of patients when participant <15 years and from the participant when age was >15 years as per institute guidelines. This study was approved by the Institute Ethics Committee.
Statistical analysis
Data are reported as a mean ± standard deviation. The categorical variables were compared using Chi-square/Fisher's exact test as per the application required. The mean values between the groups were compared using Students’ t-test. The P < 0.05 was considered statistically significant. All analyses were performed using SPSS software version 16.0 (SPSS, Chicago, IL, USA). Kaplan–Meier survival analysis was used to analyze the renal survival of patients in two CNIs groups, and log-rank test was used to compare the significance between the two groups with event as doubling of serum creatinine.
Results
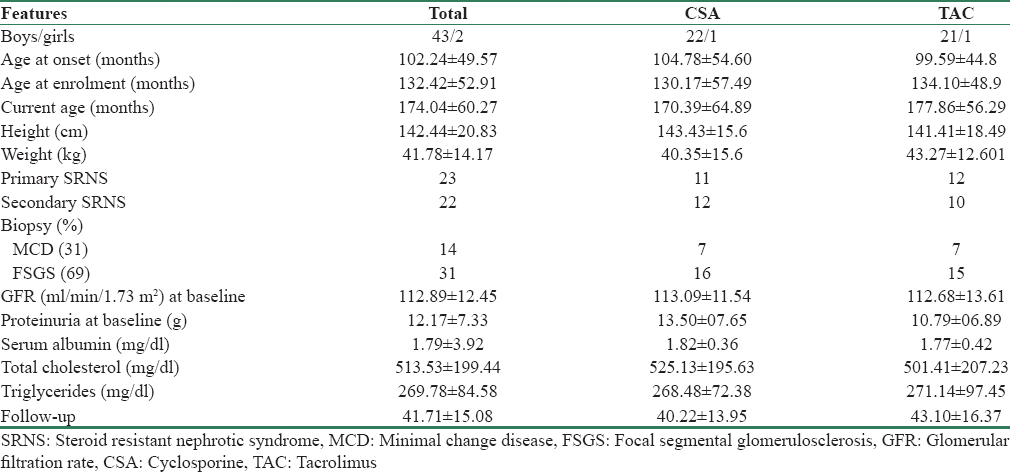
The clinical characteristics and demographic profiles of children in both CSA (n = 23) and TAC (n = 22) groups are shown in Table 1. Both groups were similar in terms of age of onset, age of enrollment in the study, gender ratio, body mass index, and eGFR. Of the 23 patients in CSA group, 7 had MCD and 16 had FSGS, and of the 22 patients in TAC group, 7 had MCD and 15 had FSGS (P = 0.9).
Primary end point
The outcomes of the patients at different periods of time are shown in Table 2. At 6 months of therapy, in the CSA group, 16 (69.5%) patients achieved either complete 13 (56.52%) or partial remission 3 (13.04%) and 7 (30.5%) patients remained resistant to CSA. In the TAC group, 18 (81.8%) patients achieved either complete 11 (50%) or partial remission 7 (31.8%) and 4 (18.2%) patients remained resistant to TAC (P = 0.3).
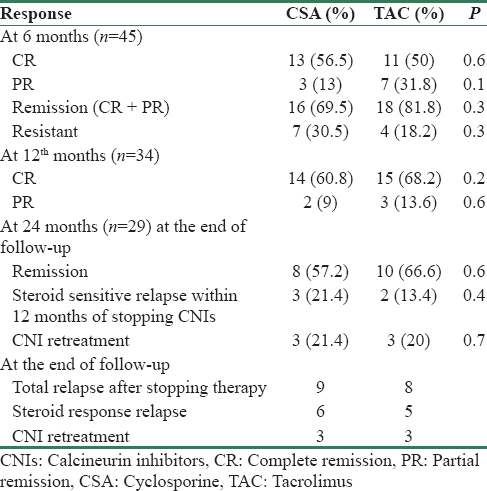
However, of the three patients with PR in CSA group, only one patient achieved CR and two did not achieve CR, and of the seven patients with PR in TAC group, four patients achieved CR and three did not achieve CR with continued therapy within the same group for 12 months. Of the 5 patients who did not achieve CR at 12 months, one patient died and four patients were further treated with MMF.
The CSA- and TAC-resistant patients were switched over from CSA to TAC and vice versa. Of the seven primary CSA-resistant patients, four achieved remission (1CR and 3 PR) and three remained resistant after switch over to TAC therapy. However, of the four primary TAC-resistant patients who were switched to CSA, only one patient showed PR and three remained resistant after switch over to CSA. All ten patients who did not have CR at 12 months of CNIs were further treated with MMF.
Secondary end points
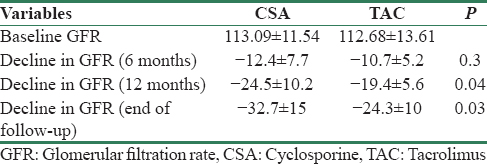
The decline in eGFR was noted in both groups of patients. The mean decline values in GFR from baseline values in CSA and TAC were not different at 6 months. However, the decline in GFR from baseline values to GFR at 12 months (P = 0.04) and at the end of follow-up (P = 0.03) was significantly greater in CSA group as compared to that of the TAC group of patients [Table 3].
On Kaplan–Meier survival analysis [Figure 2] with the event as doubling of serum creatinine, the overall mean renal survival was 57 months (95% CI: 51–64); in CSA group was 50 (95% CI: 42–64) months and in TAC group was 62 (95% CI: 55–69) months, P = 0.02. The 1-, 2-, 3-, 4-, and 5-year estimated renal survival in CSA group was 96%, 91%, 85%, 54%, and 33%, respectively, and in TAC group was 96%, 95%, 90%, 89%, and 79%, respectively. At the end of follow-up, the renal survival was only 24% in CSA group at 74 months and 53% in TAC group of patients at 72 months, respectively.
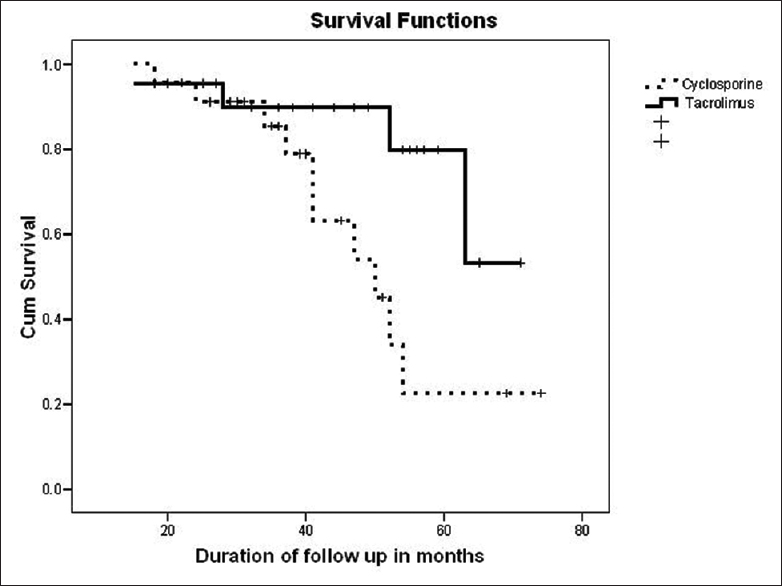
- Kaplan–Meier renal survival analysis on follow-up between cyclosporine and tacrolimus group of patients
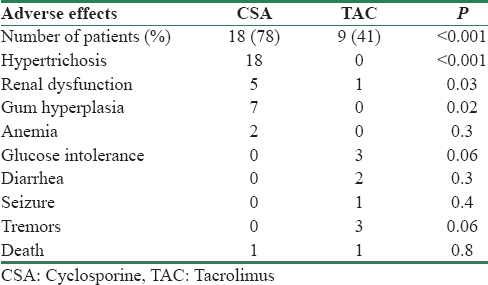
Table 4 shows the comparison of side effect profiles of the patients in both groups. Significantly, greater number of patients in CSA group (n = 18, 78%) had reported side effects as compared to the TAC (n = 9, 41%) group of patients (P < 0.001). Hypertrichosis and gum hyperplasia were significantly high in CSA group as compared to TAC group. Glucose intolerance, tremor, and seizure were unique to TAC group although statistically nonsignificant.
Relapse of nephrotic syndrome after withdrawal of calcineurin inhibitors
During the 12 months of follow-up after withdrawing CNI treatment, 8 (57.2%) and 10 (66.6%) patients were in remission in CSA and TAC group, respectively. Three patients in CSA (21.4%) and two (13.4%) in TAC group had experienced relapse; however, these relapses were steroid sensitive, 3 (21.4%) and 3 (20%) had a relapse which required retreatment with CNI, in CSA and TAC group, respectively. Till the end of follow-up, 9 (64.3%) out of 14 patients in CSA group had a relapse (6 were steroid sensitive and 3 were retreated with CSA) and 8 (53.3%) out of 15 patients in TAC group had relapse (five were steroid sensitive and three were retreated with TAC).
Effect of mycophenolate mofetil in calcineurin inhibitors nonresponsive patients on follow-up
In total, 15 patients did not achieve CR after 12 months of CNI therapy (two and three patients from CSA and TAC group, respectively, who took same CNI for 12 months and 10 patients from the switch group). Of these 15, 14 patients received treatment with MMF and 1 patient with PR on TAC died due to community-acquired pneumonia.
These 14 patients had been treated with MMF at a dose of 1200 mg/m2 for 12 months[14] and we observed that two of them had CR, five patients had PR, and seven still remained resistant. The seven patients who remained pan-resistant (i.e., CYC-SRNS with CNI and MMF resistance) were managed with enalapril and diuretics. One patient reached ESRD and one patient developed thrombosis of right femoral artery and died due to its complication.
Discussion
To the best of our knowledge, this is the single largest study which compared the efficacy of two CNIs in group of INS children who were resistant to both steroid and CYC. Our findings revealed that 16 (69.5%) children in CSA group achieved remission compared to that of 18 (81.8%) patients in TAC groups at 6 months. Furthermore, we have also observed that the TAC was more efficacious, although statistically nonsignificant, than CSA after switch over therapy from one group to another and vice versa after 6 months of therapy. The side effect profiles favor the use of TAC over CSA in this group of patients.
The decision to use the 1st CNI only for 6 months was based on a study by Loeffler et al.,[8] who reported that the maximum duration within which the patients responded to CNI was 5.5 months in their study. We have observed that only one patient who was responsive to CSA showed CR after continuation of therapy for 6 more months.
In 1993, a pilot trial of TAC as monotherapy on seven SRNS (three adults and four children) patients showed remission in six patients. Two of them had been previously treated with CYC, one with chlorambucil, and two with CSA.[7] In one of our previous study of 22 SRNS patients who received TAC, 14 of whom had been previously treated with CYC and 4 with CSA. We have found that out of 19 children who had received adequate therapy, 16 (84%) children achieved CR, and 2 (10.5%) children achieved partial remission. Three withdrew from the study and one remained nonresponsive.[13] Loeffler et al. observed CR rate of 81% and PR of 13% with TAC in a retrospective study with diverse patient population of children with IgA nephropathy, MCD, and FSGS with mixed study population consisting of only five SRNS patients and rest with steroid-dependent status. Wang et al.[15] have also studied the role of CNIs in INS which also included steroid dependent, frequently relapsing NS, along with membranous nephropathy in SRNS group of patients. They have shown that of the 34 SRNS patients, 8 received CSA and 26 TAC and TAC showed 100% while CSA showed only 50% response rate.
Our results were similar to the observations by Choudhry et al. in the only RCT with TAC versus CSA in SRNS. They showed remission in 18 (85.7%) patients in TAC group and 16 patients (80%) in CSA group, after 6 months of therapy, and concluded that TAC or CSA in combination with low-dose steroids had similar efficacy in inducing remission in children with SRNS and adverse effects in TAC group was significantly less than that of CSA group.[11] Our patient population had more severe disease as all patients were resistant to both steroid and CYC.
One of the important parts of our study was the long-term follow-up of these patients, even after withdrawing CNI therapy. It has also been observed that the patients (n = 14) who remain nonresponsive to either of CNIs at 12 months had been treated with MMF at a dose of 1200 mg/m2 for 12 months and we observed that 2 of them had CR, 5 patients had PR, and 7 remained resistant.
The exact mechanism of action of CNIs in SRNS remains speculative. The proposed mechanisms are benefits from increased immunosuppression and direct action on podocyte cytoskeleton.[1617] TAC may be beneficial even in patients who are nonresponsive to CSA. This led to further studies on the mechanism of action of TAC in SRNS. TAC is a more potent immunosuppressant than CSA.[161718] TAC suppresses cytokine production, interleukin-8 (IL-8) and IL-2 and decreases mRNA levels of granulocyte-macrophage-colony-stimulating factor, tumor necrosis factor-alpha, interferon, and c-myc in activated human peripheral blood T-cells. Thus, TAC affects growth and differentiation of T- and B-cell lymphocytes resulting in potent immunosuppression. Maruyama et al. demonstrated a significant inhibition of the production of vascular permeability factor by TAC from cultured T lymphocytes.[17] Few studies concluded that TAC exerts a pharmacological action on the transient receptor potential cation channel 6 as well.[19]
The combination of diuretics with enalapril is known to cause renal dysfunction. Patients with NS are prone to renal injury due to low intravascular volume. With this combination, renal dysfunction could occur more frequently than expected with CNI. This combination may not allow the usage of CNI for adequate duration, which may lead to false-negative end points. The GFR in patient with SRNS may be less than that estimated by serum creatinine-based formulae. Hence, in our study, the maximum dose of enalapril for controlling proteinuria and/hypertension was 50% of the maximum dose permitted for normal renal function, or in other words, the maximum permitted dose for moderate renal failure.
A recent Cochrane review on SRNS treatment concluded that further adequately powered, well-designed RCTs are needed to confirm the efficacy of CSA and to evaluate other regimens for idiopathic SRNS including high-dose steroids with CSA.[20] Although currently use of CNIs in SRNS with genetic cause is not recommended, the exact role of CNIs in this group is not known. Two cases with WT1 gene mutation benefited from CSA and one case of NPHS2 mutation responded to TAC.[21] Repeat biopsy could be of help to ascertain the cause of renal dysfunction as it may occur due to disease progression per se or due to CNI toxicity. In studies with protocol biopsies, histological features of toxicity were noted in 3.8% of the patients following 12 months therapy and 15.4%–16.7% after treatment for 12–41 months. In a retrospective study, there was no significant association between TAC exposure and biopsy changes although the average trough level was higher in those children with worsening histological findings.[222324]
Hence, our study design without the need for protocol biopsies will still remain safe to assess for CNI efficacy, provided the levels are monitored. Our study is not without limitations. A smaller number of study participants, from a single center, lack of genetic screening for specific mutation, and lack of biopsies on follow-up during CNIs therapy are the limitations that we note. Nevertheless, this is the largest study to date on CYC-SRNS, comparing CSA versus TAC in pediatric patients with a homogeneous study population. Earlier crossover to an alternative CNI in case of failure of 1st CNI, follow-up for relapse after withdrawal of CNI makes the study design unique.
Conclusion
CNIs are highly efficacious in inducing and maintaining remission in CYC-SRNS patients. Although TAC and CSA are equally efficacious, TAC has significantly less adverse effects. TAC can be even used as a salvage therapy to CYC-SRNS patients who are resistant to CSA. Larger multicenteric RCTs are needed to define the role of CNIs in CYC-SRNS patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the technical assistance of Mr. Ashok Kumar Pandey in completion of the project.
References
- Pediatric Nephrology (6th ed). Berlin, Heidelberg: Springer-Verlag; 2009. p. :667-8.
- Evidence-Based Nephrology (1st ed). Chichester, West Sussex, UK: Wiley-Blackwell; 2009. p. :787.
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2012;2(Suppl 2):139-274.
- [Google Scholar]
- Handbook of Kidney Transplantation (4th ed). Philadelphia: Lippincott Williams & Wilkins; 2004. p. :415-6.
- Clinical and mechanistic differences between FK506 (tacrolimus) and cyclosporin A. Nephrol Dial Transplant. 2000;15:1916-8.
- [Google Scholar]
- Pilot trial of FK 506 in the management of steroid-resistant nephrotic syndrome. Nephrol Dial Transplant. 1993;8:1286-90.
- [Google Scholar]
- Tacrolimus therapy in pediatric patients with treatment-resistant nephrotic syndrome. Pediatr Nephrol. 2004;19:281-7.
- [Google Scholar]
- Experience with tacrolimus in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2009;24:1517-23.
- [Google Scholar]
- Treatment outcome of late steroid-resistant nephrotic syndrome: A study by the Midwest Pediatric Nephrology Consortium. Pediatr Nephrol. 2013;28:1235-41.
- [Google Scholar]
- Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: A randomized controlled trial. Am J Kidney Dis. 2009;53:760-9.
- [Google Scholar]
- The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571-90.
- [Google Scholar]
- Tacrolimus: A new therapy for steroid-resistant nephrotic syndrome in children. Nephrol Dial Transplant. 2008;23:910-3.
- [Google Scholar]
- Mycophenolate mofetil in treatment of childhood steroid-resistant nephrotic syndrome. J Nephrol. 2011;24:203-7.
- [Google Scholar]
- Treatment of tacrolimus or cyclosporine A in children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27:2073-9.
- [Google Scholar]
- Differing proteinuria control with cyclosporin and tacrolimus. Lancet. 1997;349:330.
- [Google Scholar]
- Use of tacrolimus in steroid- and cyclophosphamide-resistant minimal change nephrotic syndrome. Am J Kidney Dis. 2003;42:E13-5.
- [Google Scholar]
- FK 506 for vascular permeability factor production in minimal change nephrotic syndrome. Nephron. 1994;66:486-7.
- [Google Scholar]
- Treatment of focal segmental glomerulosclerosis with immunophilin modulation: When did we stop thinking about pathogenesis? Kidney Int. 2009;76:487-91.
- [Google Scholar]
- Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev. 2010;11:CD003594.
- [Google Scholar]
- Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2010;5:2075-84.
- [Google Scholar]
- Long-term cyclosporine therapy for pediatric nephrotic syndrome: A clinical and histologic analysis. J Am Soc Nephrol. 1996;7:543-9.
- [Google Scholar]
- Follow-up study of children with nephrotic syndrome treated with a long-term moderate dose of cyclosporine. Am J Kidney Dis. 1998;31:932-9.
- [Google Scholar]
- Calcineurin inhibitor induced nephrotoxicity in steroid resistant nephrotic syndrome. Indian J Nephrol. 2013;23:41-6.
- [Google Scholar]







