Translate this page into:
Clinicopathological Study of Males with Lupus Nephritis: Pathologist's Experience at a Tertiary-Care Center
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Systemic lupus erythematosus (SLE) is an autoimmune systemic disorder, more common in females of reproductive age-group as compared with males. There are very few studies regarding lupus nephritis (LN) in males. Hence, we decided to study the clinical and pathological findings of LN in males.
Materials and Methods:
We carried out a retrospective study over a period of 5 years (January 2014–December 2018) on indicated native renal biopsies from male patients with LN. We analyzed the clinical, laboratory, and histological findings of these patients.
Results:
Renal biopsies were performed on 228 patients with LN, of which 29 (12.72%) biopsies were in male patients. The mean age at presentation was 28.3 ± 12.98 years. Edema (65.5%) was the most common clinical feature followed by arthritis (27.58%), fever (27.58%), and skin rash (24.1%). The mean values for 24 hours urinary protein, serum double-stranded DNA, serum antinuclear antibody, and serum complement C3 were 4.98 ± 2.91 g, 137.7 ± 91.93 IU/mL, 2.96 ± 1.78, and 65.07 ± 36.30 mg/dL, respectively. On histology, the most common class of LN was Class IV (34.48%) followed by Class V (20.68%), combined Class IV + V (20.68%), Classes II, III, and III + V.
Conclusion:
LN can affect males, although the prevalence is lower than in females. The incidence of LN in our study was 12.7% with the most common histological class being diffuse proliferative LN.
Keywords
Diffuse proliferative
hypocomplementemia
lupus nephritis
males
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease, often called a “woman's disease” because of the high prevalence of SLE in females.[12] SLE can involve any organ, and when it affects the kidneys, it is known as “lupus nephritis” (LN). LN can manifest with minimal renal involvement in the form of mild proteinuria to severe end-stage renal disease (ESRD). Nearly 5% to 22% of these patients progress to ESRD.[34] SLE is common during childbearing age in females, and males are usually affected in their adolescence.[56] The main etiological factors considered are sex hormones and immunological factors. The criteria laid down by the American College of Rheumatology for SLE are used worldwide for diagnosis and disease activity.[78] Renal biopsy is still the gold standard for diagnosis, and prognosis of LN and biopsy findings are reported according to the classification system by the International Society of Nephrology (ISN) and the Renal Pathology Society (RPS).[9] In this study, we analyzed the clinical, laboratory, and biopsy findings in male patients with LN.
Materials and Methods
This was an Institutional Review Board–approved, single-center, retrospective study of indicated renal biopsies performed on male patients with SLE between January 2014 and December 2018. The patient demography included age, sex, and clinical features. The laboratory parameters analyzed were 24 hours urinary protein (g), serum creatinine (SCr; mg/dL), serum anti-double-stranded deoxynucleotide antibody (anti-dsDNA; IU/mL), serum antinuclear antibody (S.ANA), and serum complement C3 (S.C3; mg/dL). Anti-dsDNA antibodies (titer <25 IU/mL: negative) were determined by immunometric enzyme immunoassay (AutoBind, Tosoh India Pvt. Ltd.), S. ANA (<1: negative; >1: positive) by immunometric enzyme immunoassay (AutoBind, Tosoh India Pvt. Ltd), and S.C3 (normal range: 90–207 mg/dL) by quantitative, turbidimetric assay (Siemens, Germany).
For light microscopy, formalin-fixed, paraffin-embedded sections of 3 to 4 μm thickness were stained with hematoxylin and eosin, periodic acid–Schiff, Jones's methenamine silver, and Gomori's trichrome stains. Immunofluorescence (IF) study was performed on 4 to 5 μm thick frozen sections cut on cryostat and stained with antihuman IgG, IgA, IgM, C3, C1q, and fibrinogen (polyclonal antihuman rabbit antisera; conjugated to fluorescein isothiocyanate, supplied by Dako, USA). The biopsies were studied for changes in the glomerulus, blood vessels, and tubulointerstitial compartment and were reported according to the histological classification as per the ISN/RPS 2003 classification of LN. The activity and chronicity scoring of LN was also calculated as per the ISN/RPS 2003 classification.[9] Five pathologists independently reported, and the consensus diagnosis generated was finally reported.
Results
Of the 228 biopsy-proven LN patients, 29 (12.72%) were males (study group) and 199 (87.2%) were females (male: female = 1:14.5). The mean age at presentation was 28.3 ± 12.98 years. All patients (100%) had nephrotic–nephritic presentation. Other demographic details are shown in Table 1. Hematological investigation revealed anemia (100%) and thrombocytopenia and leukocytopenia (3.45%). The mean 24 hours urinary protein was 4.98 ± 2.91 g, and 13 (44.82%) patients presented with raised SCr (mean: 1.70 ± 1.30 mg/dL). The mean dsDNA was 137.7 ± 91.93 IU/mL and S. ANA was positive in 27 (93.1%) patients (mean: 2.96 ± 1.78). Hypocomplementemia was observed in 22 (75.86%) patients (mean: 65.07 ± 36.30 mg/dL). Table 2 presents the demographic characteristics with respect to histological class.
| Clinical Manifestation | Present study, n=29 | Liu et al.,[25] n=85 | Wang et al.,[24] n=45 |
|---|---|---|---|
| Edema | 19 (65.51%) | - | - |
| Hematuria | 15 (51.72%) | 57 (67.1%) | 33 (73.3%) |
| Fever | 8 (27.58%) | 42 (49.4%) | 17 (37.8%) |
| Arthritis | 8 (27.58%) | 31 (36.5%) | 26 (57.8%) |
| Skin rash | 7 (24.14%) | 42 (49.4%) | 26 (57.8%) |
| Serositis | 4 (13.79%) | 43 (50.6%) | 7 (15.6%) |
| Oliguria | 3 (10.34%) | - | - |
| Central Nervous system | 1 (3.45%) | 2 (2.4%) | 2 (4.4%) |
| Leucopenia | 1 (3.45%) | 26 (30.6%) | 25 (55.6%) |
| Thrombocytopenia | 1 (3.45%) | 27 (31.8%) | 12 (27.3%) |
| Alopecia | - | 18 (21.2%) | 6 (13.3%) |
| Oral ulcer | - | 15 (17.6%) | 13 (28.9%) |
| Total No., n=29 | Class II, n=3 (10.34%) | Class III, n=2 (6.89%) | Class IV, n=10 (34.48%) | Class V, n=6 (20.68%) | Class IV + V, n=6 (20.68%) | Class III + V, n=2 (6.89%) |
|---|---|---|---|---|---|---|
| Low C3 (≤90 mg/dL) | 2 (66.66) | 2 (100) | 8 (80) | 3 (50) | 5 (83.3) | 2 (100) |
| S.dsDNA (≥25 IU/mL) | 3 (100) | 2 (100) | 10 (100) | 6 (100) | 6 (100) | 2 (100) |
| S.ANA titer (≥1) | 2 (66.6) | 2 (100) | 10 (100) | 5 (83.3) | 6 (100) | 2 (100) |
| S. Cr (≥1.4 mg/dL) | - | - | 7 (70) | 2 (40) | 3 (50) | 1 (50) |
| 24 h urinary proteins (g) (>3.5) | 2 (66.66) | - | 7 (70) | 4 (80) | 5 (83.3) | 1 (50) |
| 24 h urinary proteins (g) (<3.5 but >1.5) | 1 (33.3) | 2 (100) | 3 (30) | 2 (40) | 1 (16.66) | 1 (50) |
S. dsDNA=serum double-stranded deoxynucleotide antibody, S.ANA=Antinuclear antibody, S. Cr=serum creatinine
Light microscopy findings
Table 2 presents the demographic characteristics with respect to histological class. None of the biopsies were reported to have histological changes of Class I/VI LN. Class II LN was reported on three (10.34%) biopsies [Figure 1]. Of these, 2 (66.66%) patients presented with nephrotic-range proteinuria (mean: 6.18 ± 2.04 g/day), and one (33.33%) had subnephrotic-range (1.71 g/day) proteinuria. Serum dsDNA (S. dsDNA) was high in all three cases, whereas S. ANA was positive only in 2 cases. The mean S.Cr was 0.89 ± 0.33 mg/dL.

- Light microscopy of a renal biopsy from a patient with Class II lupus nephritis. The glomerulus appears mildly enlarged with mild mesangial expansion and mild hypercellularity. Hematoxylin & eosin, magnification 200×
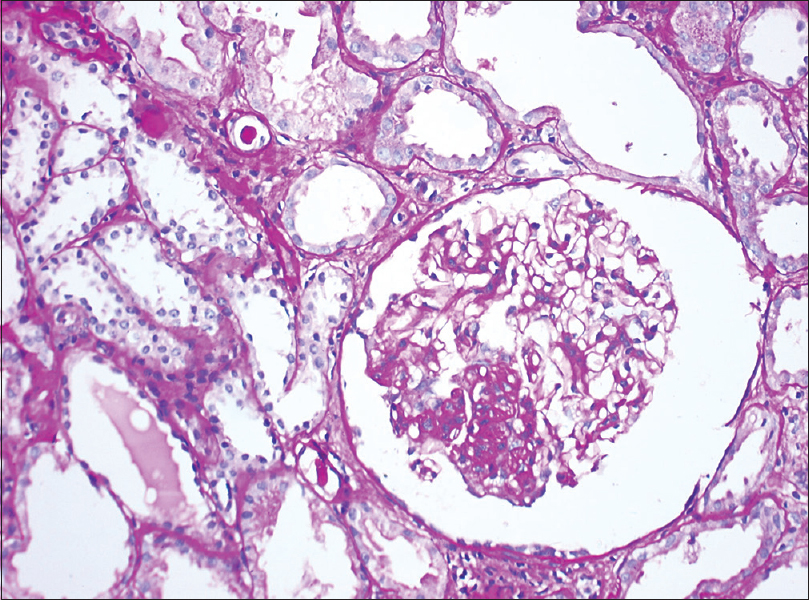
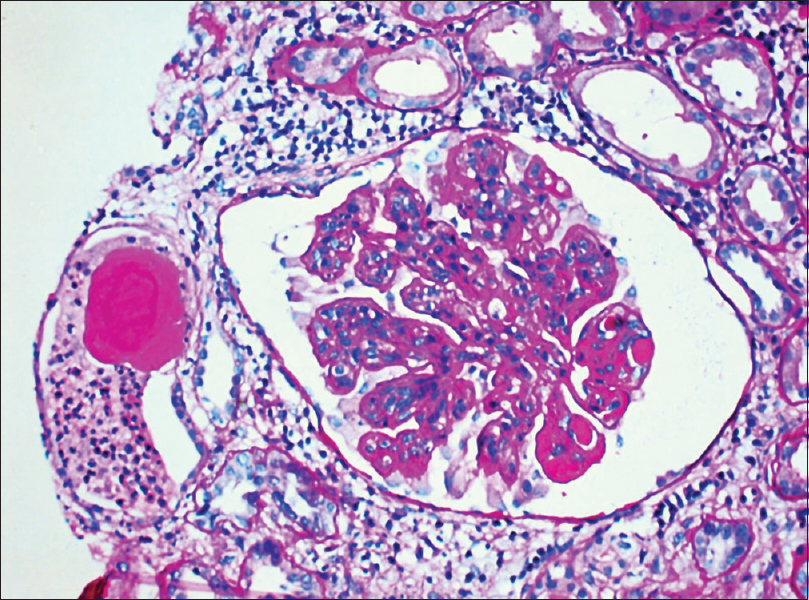
Class III LN was reported in 2 biopsies with mild tubulointersitial nephritis (TIN) and focal interstitial fibrosis in one biopsy [Figure 2]. Mean S. Cr was 0.73 ± 0.52 mg/dL. Ten (34.48%) biopsies revealed Class IV LN. This was the most commonly reported class. Histology revealed endocapillary proliferation (100%), neutrophilic infiltration (60%), wire loop lesions (60%), and hyaline thrombi (50%). Cellular crescents were noted in seven (70%), fibrous crescents in two (20%), and fibrinoid necrosis in one (10%) [Figure 3]. Class IV LN (G/A) was noted in 70% and Class IV (A/C) in 30% biopsies. Chronicity in the form of glomerular sclerosis, tubular atrophy with interstitial fibrosis, and chronic TIN was seen in three (30%), four (40%), and eight (80%) biopsies. Seven (70%) patients had a mean S. Cr of 2.53 ± 1.31 mg/dL with nephrotic-range (mean: 7.79 ± 2.59 g/day) and remaining three (30%) with subnephrotic-range (mean: 2.33 ± 0.45 g/day) proteinuria. All the patients revealed high S. dsDNA values. S. ANA was positive in all the patients of this class, and hypocomplementemia was seen in eight (80%) patients. Lupus vasculopathy was noted in one patient of this class.
- Light microscopy of a renal biopsy from a patient with Class III lupus nephritis. The glomerulus reveals focal sclerosing glomerulonephritis and focal hypercellularity. Periodic acid–Schiff, magnification 200×
- Light microscopy of a renal biopsy from a patient with Class IV lupus nephritis. The glomerulus showing hyaline thrombi, dilated capillary lumina, and mild mesangial hypercellularity. (a) Periodic acid–Schiff, magnification 400×
Class V LN was reported in 6 (20.68%) cases with light microscopy changes of diffuse thickening of glomerular basement membranes with subepithelial spikes. Two (33.33%) revealed neutrophilic infiltration and wire loop lesions and 1 showed endocapillary proliferation. TIN was noted in 3 and interstitial fibrosis and tubular atrophy (IFTA) in 2 biopsies. Nephrotic-range (mean: 6.29 ± 1.53 g/day) proteinuria was noted in 4 and subnephrotic-range (mean: 2.38 ± 0.25 g/day) proteinuria in 2. All patients had high values for S. dsDNA of which positive S. ANA was observed in five (83.3%) cases. Three (50%) patients had low S. C3 and mean S. Cr was 1.10 ± 0.49 mg/dL.
Combined lesions
A mixed class (27.58% biopsies) was diagnosed when histological features of two classes were observed in the same biopsy core. The most common combined class was Class IV + V seen in 6 with 4 biopsies showing capillary lumina with neutrophils and hyaline thrombi. Cellular crescents were seen in 3 and fibrinoid necrosis in one biopsy. IFTA was seen in 3 and glomerulosclerosis in 2 biopsies [Figure 4]. Five (83.3%) patients presented with nephrotic-range proteinuria (mean: 5.74 ± 2.52 g/day). Raised S. dsDNA and positive S. ANA were noted in all the patients of this group. Hypocomplementemia was seen in five (83.3%) patients, and the mean S. Cr of patients with this class was 1.52 ± 0.67 mg/dL.

- Light microscopy of a renal biopsy from a patient with Class IV + V lupus nephritis. The glomerulus showing lobular accentuation with capillary lumina lined by thickened membranes (wire loop lesion). (A) Periodic acid–Schiff, magnification 200×
Of 2 biopsies of Class III + V, one showed hyaline thrombi with fibrinoid necrosis. Both the patients had high S. dsDNA titer, positive S. ANA, and hypocomplementemia. One patient presented with nephrotic-range proteinuria and the other with subnephrotic-range proteinuria. Mean S. Cr was 1.14 ± 0.50 mg/dL.
Immunofluorescence microscopy findings
Seventeen (58.62%) biopsies revealed full-house positivity for immunoglobulins and complements on IF microscopy. IgG (intensity of +3 or +4 on the scale of 0 to +4) was noted in all the biopsies, followed by C1q (96.5%). Anti-IgA and anti-IgM were noted in 82.7% and C3 in 72.41% biopsies. IgA of +2 intensity was noted in 15 (51.72%) cases and of +3 in 14 (48.27%) cases. The intensity of IgM was variable, 10 (34.48%) biopsies revealed mesangial IgM deposit of +1 intensity, 11 (37.93%) of +2, and eight (27.58%) with +3 intensity.
Uniform presence of complements was seen in all the cases. C3 of +3 intensity was present in nine (31.03%) and of +4 intensity in 11 (37.93%) biopsies. An intensity of +3 for C1q was noted in 21 (72.41%) biopsies. Eight (27.58%) biopsies revealed the presence of C1q antibodies of +4 intensity [Figure 5]. The distribution of deposits was variable in each class. In Classes II, III, and IV, the deposits were mainly mesangial, whereas in Class V, the deposits were mainly along the glomerular capillary walls and also in the mesangium. IgG was noted along the tubular basement membrane in 26% of the biopsies and one case of lupus vasculopathy revealed the presence of IgG (+2) and fibrinogen (+2) in the vessel wall.

- Immunofluorescence findings in an index case of lupus nephritis with immunoglobulins and complement
Discussion
LN can occur at any time during the course of SLE with implicated factors such as genetics, sex hormones, and autoimmunity. The relation between estrogen and autoimmune disease has been hypothesized for a high incidence of SLE in females. Dysregulation of T-cells, increased helper T-cell activity, and B-cell hyperactivity lead to the generation of autoantibodies.[101112131415] Shen et al.[16] found a strong association of TLR7 with SLE in Eastern Asian males than in females. In males, the androgen hormone is responsible for renal injury.[17]
Nearly 25% to 75% of SLE patients present with LN.[131819] The incidence of LN in males varies from 4% to 22% with an incidence of 12.7%.[120] Basu et al.[21] and de Carvalho et al.[22] reported an incidence of male lupus of 9.67% and 13.6%, respectively. Patel et al.[23] reported a high prevalence of male LN with increasing age. The mean age at presentation was 28.3 ± 12.98 years (range: 10–55 years) in our study, which was comparable with 42.6 years by Basu et al.,[21] 32.67 ± 13.96 years by Wang et al.[24] and 37.94 ± 16.96 years (10–87 years) by Liu et al.[25] Male LN was common in the age-group of 20 to 40 years in the present study, Basu et al.[21] (40–48 years), and Wang et al.[24] (18–46 years).
The most common presenting symptom was edema 19 (65.5%) followed by hematuria 15 (51.72%) in the present study. Arthritis and fever were noted each in 8 (27.58%) patients. Skin rashes in 7 (24.14%), serositis in 4 (13.79%), and oliguria in 3 (10.34%) patients. Low incidence of arthritis in males has also been reported by Faezi et al.[26] Medina et al.,[27] de Carvalho et al.,[22] and Liu et al.[25] reported a higher incidence of skin rash of 84%, 45.5%, 49.4%, respectively. We also reported a low incidence (13.79%) of serositis in our study, which was more common in the study by Voulgari et al.,[28] Soto et al.,[29] and Keskin et al.[30] Some studies reported that male patients with LN have more of neurological involvement,[293132] vascular thrombosis,[3233] cardiovascular damage, and hypertension;[34] however, we did not observe these.
Nephrotic-range proteinuria was observed in 19 (65.51%) patients (mean range: 4.98 ± 2.91 g), similar to the findings of Wang et al.,[24] Hsu et al.,[35] and Urrestsarzú et al.[5] who reported a mean 24 hours urinary protein (in g) of 5.26 (2.63–9.90), 5.0 (2.7–9.1), and 4.6 ± 3.5, respectively. Nephrotic-range proteinuria was more common in Classes II, IV, V, and IV + V similar to the observation by Liu et al.[25]
All the patients in our study revealed high anti-dsDNA titer. S. ANA was positive in 27 (93.1%) cases. Wang et al.[24] reported high anti-dsDNA titer and positive S.ANA in 64.4% and 74% patients, respectively. The results obtained by Urrestsarzú et al.[5] were also similar. They also observed high serum anti-dsDNA and S.ANA in 96.0% and 97.8% patients, respectively.[5]
The mean S.Cr in our study was 1.70 ± 1.30 mg/dL. High S.Cr (>1.4 mg/dL) was noted in 13 (44.82%) patients in our study, of which 7 (70%) were from Class IV, 2 from Class V, 3 from Class IV + V, and one from Class III + V. Mean S. Cr of patients with biopsy changes of Classes II and III was <1.4 mg/dL. High S. Cr levels were also reported in the study by Urrestsarzú et al.[5] and de Carvalho et al.[22] (2.18 ± 1.47 and 3.16 ± 2.49 mg/dL, respectively). Proteinuria and S. Cr have been considered a significant overall prognostic parameter for the disease.[36]
Very few authors have exclusively reviewed the histological parameters of LN in males. The results of our study are comparable with the studies by Wang et al.,[24] Liu et al.,[25] and de Carvalho et al.[22] Most of these studies show that Classes IV and V are the most common histological classes reported in male patients with LN. In our study also, we found that the most common class on histology was Class IV (n = 10; 34.48%). Class V and combined Class IV + V were next common to be reported in six (20.68%) patients. A high incidence of Class IV LN was also noted by Wang et al.[24] (60%), Liu et al.[25] (35.7%), and de Carvalho et al.[22] (36.4%). However, de Carvalho et al.[22] reported a higher incidence of Class V of 45.5%. The incidence of other classes in descending trend in our study was Class II (10.34%), Class III (6.89%), and Class III + V (6.89%), a comparison of which with other studies is summarized in Table 3.
| ISN/RPS CLASS | Present study, n=29 | Wang et al.,[24] n=45 | Liu et al.,[25] n=42 | de Carvalho,[22] n=11 |
|---|---|---|---|---|
| Class II | 3 (10.3%) | 3 (6.7%) | 1 (2.7%) | - |
| Class III | 2 (6.89%) | 7 (15.6%) | 5 (11.9%) | 2 (18.2%) |
| Class IV | 10 (34.48%) | 26+1 (60%) | 15 (35.7%) | 4 (36.4%) |
| Class V | 6 (20.68%) | 8 (17.8%) | 10 (23.8%) | 5 (45.5%) |
| Class IV + V | 6 (20.68%) | - | 10 (23.8%) | - |
| Class III + V | 2 (6.89%) | - | 1 (2.4%) | - |
ISN=International Society of Nephrology, RPS=Renal Pathology Society
We also analyzed the histological features seen on the biopsy, which was not done by any author previously. However, Hsu et al.[35] did compare the activity and chronicity scores. The most common histological finding in our study was endocapillary proliferation, which was observed in 20 (68.96%) biopsies. The infiltration of glomeruli by neutrophils was seen in 12 (41.37%) cases and was the next common microscopic finding noted in our study. Hyaline thrombi were characteristically seen in 11 (37.93%), cellular crescents in 10 (34.48%), and wire loop lesions in eight (27.58%) biopsies. The least common finding in glomeruli was the presence of fibrinoid necrosis in three (8.7%) and the presence of fibrous crescents in two (6.89%) biopsies. Hsu et al.[35] reported the presence of fibrinoid necrosis in 88.2% and the presence of cellular crescents on biopsy in 47.1% of cases of male LN. Tubular atrophy was noted in 10 (34.48%) biopsies and was more so noted in cases of Class IV LN. Similarly, interstitial fibrosis was more common in biopsies with changes of Class IV LN (4; 40%). Interstitial inflammation of variable degree was associated with all the classes; however, most of the cases of Class IV (8; 80%) revealed associated interstitial nephritis. Thus, renal involvement with nephrotic-nephritic presentation is also similarly noted in males with SLE.
Limitations of the study
Because of the unavailability of electron microscopy, the biopsies were not subjected to this modality. We did not follow up with the patients for change of class as we aimed at studying the histological and clinical profile of male patients with LN, hence it is not in the scope of this article.
Conclusion
SLE does affect the males, but with a lower incidence. The spectrum of histological changes are similar irrespective of sex.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Review: Male systemic lupus erythematosus: A review of sex disparities in this disease. Lupus. 2010;19:119-29.
- [Google Scholar]
- Systemic lupus erythematosus in males: A case series. Saudi J Kidney Dis Transpl. 2014;25:638-42.
- [Google Scholar]
- Demographic differences in the development of lupus nephritis: A retrospective analysis. Am J Med. 2002;15(112):726-9.
- [Google Scholar]
- Review: Lupus nephritis: Pathologic features, epidemiology and a guide to therapeutic decisions. Lupus. 2010;19:557-74.
- [Google Scholar]
- Lupus nephritis in males: Clinical features, course, and prognostic factors for end-stage renal disease. Kidney Int Rep. 2017;2:905-12.
- [Google Scholar]
- Prognostic determinants in lupus nephritis: A long-term clinicopathologic study. Lupus. 1995;4:109-15.
- [Google Scholar]
- 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-12.
- [Google Scholar]
- The American College of Rheumatology criteria for the classification of systemic lupus erythematosus: Strengths, weaknesses, and opportunities for improvement. Lupus. 1999;8:586-95.
- [Google Scholar]
- The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241-50.
- [Google Scholar]
- Gender disparity in systemic lupus erythematosus, thoughts after the 8th International Congress on Systemic Lupus Erythematosus, Shanghai, Chaina, 2007. J Clin Rheumatol. 2008;14:185-7.
- [Google Scholar]
- Sex hormones and systemic lupus erythematosus: Review and meta-analysis. Arthritis Rheum. 2003;48:2100-10.
- [Google Scholar]
- Hormonal modulation of B cell development and repertoire selection. Mol Immunol. 2005;42:811-20.
- [Google Scholar]
- Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669-72.
- [Google Scholar]
- Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181:1556-62.
- [Google Scholar]
- Sex-specific association of X-linked Toll-like receptor 7(TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107:15838-43.
- [Google Scholar]
- Gender and the progression of chronic renal diseases: Does apoptosis make the difference? Minerva Urol Nefrol. 2004;56:1-14.
- [Google Scholar]
- Clusters of clinical and immunologic features in systemic lupus erythematosus: Analysis of 600 patients from a single center. Semin Arthritis Rheum. 2004;33:217-30.
- [Google Scholar]
- Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestation in acohort of 1000 patients. Medicine (Baltimore). 2003;82:299-308.
- [Google Scholar]
- Systemic lupus erythematosus in males.A study of 107 Latin American patients. Medicine (Baltimore). 1996;75:124-30.
- [Google Scholar]
- Native renal biopsy: An essential diagnostic tool in systemic lupus erythematosus. Ann Pathol Lab Med. 2018;5:647-52.
- [Google Scholar]
- The prevalence and incidence of biopsy-proven lupus nephritis in the UK. Arthritis Rheum. 2006;54:2963-9.
- [Google Scholar]
- Clinicopathological characteristics and outcomes of male lupus nephritis in China. Lupus. 2012;21:1472-81.
- [Google Scholar]
- Clinical and pathological characteristics of male patients with systemic lupus erythematosus from northeast China: A ten-year retrospective study. Int J Clin Exp Pathol. 2017;10:6082-91.
- [Google Scholar]
- Clinical and immunological pattern of systemic lupus erythematosus in men in a cohort of 2355 patients. J Rheum Dis. 2014;17:394-9.
- [Google Scholar]
- Clinical spectrum of males with primary antiphospholipid syndrome and systemic lupus erythematosus: A comparative study of 73 patients. Lupus. 2004;13:11-6.
- [Google Scholar]
- Gender and age differences in systemic lupus erythematosus.A study of 489 Greek patients with a review of the literature. Lupus. 2002;11:722-9.
- [Google Scholar]
- Systemic lupus erythematosus in 49 Israeli males: A retrospective study. Clin Exp Rheumatol. 1987;5:233-40.
- [Google Scholar]
- Systemic lupus erythematosus disease severity in men and women: A case control study. J Rheumatol. 2002;29:1674-7.
- [Google Scholar]
- Systemic lupus erythematosus in men–A different prognosis? Z Rheumatol. 1994;53:339-45.
- [Google Scholar]
- Male systemic lupus erythematosus in a Latin-American inception cohort of 1214 patients. Lupus. 2005;14:938-46.
- [Google Scholar]
- Age-and gender related long term renal outcome in patients with lupus nephritis. Lupus. 2011;20:1135-41.
- [Google Scholar]
- Predictors of renal outcome in diffuse proliferative lupus nephropathy: Data from repeat renal biopsy. Nephrol Dial Transplant. 2000;15:1604-8.
- [Google Scholar]







