Translate this page into:
Post Covishield (ChAdOx1 nCoV-19) Vaccination: New Onset Focal Segmental Glomerulosclerosis Resistant to Steroid and Calcineurin Inhibitor
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
With the ongoing mass COVID vaccination program, various case reports link the COVID-19 vaccines with heightened off-target immune responses leading to de novo development or relapse of various glomerular diseases. Very few glomerular diseases (totally nine published cases to date) have been reported post ChAdOx1 nCoV-19 (Oxford–AstraZeneca) vaccination compared to more potent m RNA vaccine. In this case report, we present a case of de novo focal segmental glomerulosclerosis (FSGS) post ChAdOx1 nCoV-19 vaccination resistant to steroid and calcineurin inhibitor treatment. To our knowledge, this is the first case of FSGS tip variant reported after the ChAdOx1 nCoV-19 vaccination and the second de novo FSGS case post COVID vaccination (any types of COVID vaccines). We may expect more such types of cases resistant to conventional therapy as the global penetration of vaccination programs will improve.
Keywords
ChAdOx1 nCoV-19 vaccine
focal segmental glomerulosclerosis
glomerulonephritis
Introduction
The COVID vaccination drive commenced on Jan 16, 2021, in India with vaccination to all health care workers. The program was expanded with time to include vaccination of front-line workers, citizens more than 60 years of age, citizens more than 45 years of age, citizens more than 18 years of age, and eventually citizens 15–18 years of age. India has three available vaccines as of now [Covishield (ChAdOx1 nCoV-19; manufactured by Serum Institute of India), Covaxin (BBV152; Bharat Biotech), and Sputnik V (Gam-COVID-Vac; Gamaleya Research Institute of Epidemiology and Microbiology)] approved for emergency use. As a consequence of pro-active implementation, more than 141 crore COVID vaccination doses have been administered, with 90% of the adult population of the country covered with at least one dose and 62% of the adult population with both the doses.[1] This deployment of mass vaccination all over the world has also raised new concerns for nephrologists as various case reports link the COVID-19 vaccines with heightened off-target immune responses leading to de novo development or relapse of various glomerular diseases. In this case report, we present a case of nephrotic syndrome—biopsy-proven focal segmental glomerulosclerosis tip variant (FSGS-T), developed 12 days after the first dose of COVID-19 vaccination with Covishield [ChAdOx1 nCoV-19]. Despite having been put on steroid, calcineurin inhibitor, and rituximab, he has not shown any response till now and still has features suggestive of nephrotic syndrome for 11 months of disease onset.
Case Report
A 21-year-old male presented with facial puffiness and lower limb swelling of 10 days duration in mid-February 2021. He had a history of Covishield [ChAdOx1 nCoV-19] vaccination first dose in the last week of January around 12 days before the onset of these symptoms. He had no history of oliguria, hematuria, lithuria, or any other systemic complaints. There was no history of alternative drugs intake. He had no family history of renal disease. Initial investigations revealed normal complete blood count, urine examination/microscopic examination: protein 4+, 24-h urinary protein of 23.87 g, serum creatinine 1.06 mg/dl, serum albumin 0.9 g/dl, and total cholesterol 324 mg/dl. Ultrasound abdomen revealed a normal-sized kidney with mild ascites. His autoimmune markers [antinuclear antibodies (ANA), anti-dsDNA, antineutrophil cytoplasmic antibodies (ANCA)] were negative with a normal complement (C3/C4) level. Viral serology (HIV, HBsAg, anti-HCV) was negative. His COVID-19 RT-PCR was negative and anti-spike SARS CoV-2 IgG antibody quantitative (chemiluminescent microparticle immunoassay) was 667.90 AU/ml (ref. interval <50). He underwent a renal biopsy which revealed light microscopy-21 glomeruli, none globally sclerosed. The glomeruli appeared viably enlarged and revealed focal dilatation and congestion of capillary lumina. Mild segmental increase in the mesangial matrix was noted in a few tufts. Three (14.28%) glomeruli showed segmental tuft sclerosis with focal intraglomerular foam cell changes involving the tip region of the capillary tuft in two glomeruli. Tubular atrophy and interstitial fibrosis involved less than 10% of sampled cortex. The final impression was FSGS-T involving 3/21 (14.28%) of sampled glomeruli [Figure 1]. Direct immunofluorescence was negative for all immunoreactants. Electron microscopy revealed diffuse effacement of visceral epithelial foot processes. No electron-dense/organized deposits were noted in the mesangial areas or glomerular basement membrane [Figure 2].
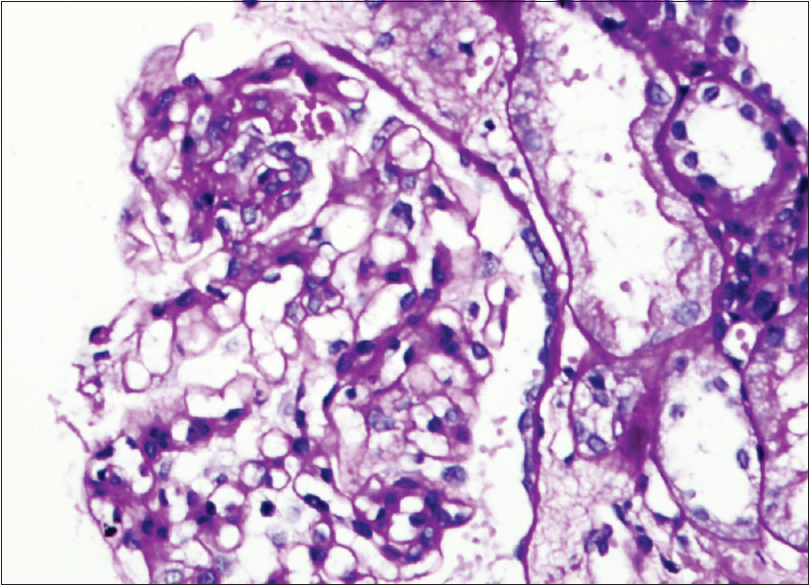
- Light microscopy image: Photomicrograph showing segmental glomerular sclerosis (PAS X200)
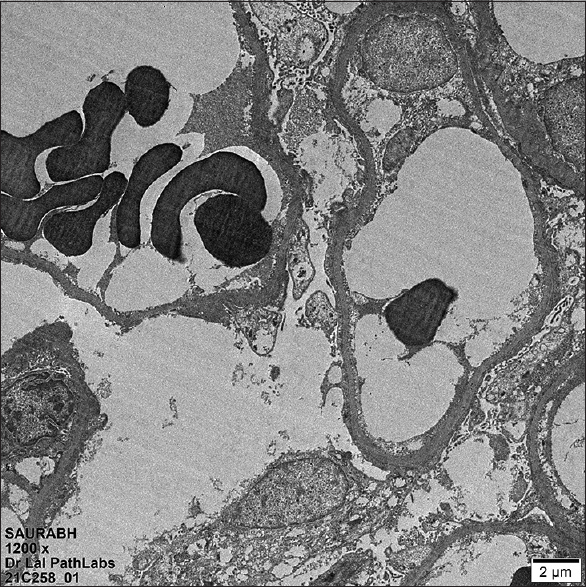
- Transmission electron microscopy (TEM) image showing diffuse effacement of visceral epithelial cell foot processes (TEM X1200)
He was put initially on steroid (Tab. prednisolone 70 mg/day, weight 72 kg), diuretics, angiotensin receptor blocker (ARB), statins, low molecular weight heparin, and other supportive measures. He had mild renal dysfunction (serum creatinine increased from 1.06 mg/dl to 1.9 mg/dl) within a few days of treatment initiation which settled to baseline after reducing the dose of diuretic and ARB. Even after the continuation of the steroid for 12 weeks, he remained severely hypoalbuminemic and had massive nephrotic range proteinuria (24-h urinary protein of 18 g to 16 g/day). He also developed clinical features suggestive of steroid toxicity. It was decided to put on calcineurin-based therapy—tacrolimus (started with 3 mg twice a day) and the dose titrated to maintain the tacrolimus level (5–10 ng/ml) as per institution protocol with a tapering dose of steroid thereafter. The last follow-up tacrolimus trough level was 8 ng/ml. He had no response even after about seven months of tacrolimus along with a low dose of steroid (10 mg prednisolone/day) with persisting nephrotic range proteinuria (24-h urinary protein of 16 g/day) and hypoalbuminemia. Follow-up investigations are presented in Table 1. In the last week of November 2021, he was administered four doses of Inj. rituximab with the continuation of tacrolimus, low-dose steroid, and ARB. CD-19 (total B-cells) in flow cytometry single-platform bead assay done 4 weeks post rituximab infusion was 0% (3–20%). As of now, he has shown no response to all these treatments. He is maintaining normal serum creatinine with all the biochemical parameters (severe hypoalbuminemia and hypercholesterolemia), suggestive of nephrotic syndrome. Nephrotic syndrome gene panel (of about 61 genes including ACTN4, TRPC6, INF2, NPHS1, NPHS2, CD2AP, MYO1E, PLCE1, APOL1, CFH, LMX1B, etc.) from MEDGENOME done by next-generation sequencing was negative. As the rituximab response may take some time, we are continuing with tacrolimus, low-dose steroid, ARB, and other supportive measures.
| Period of investigation | Serum creatinine (mg/dl) | Serum albumin (g/dl) | Urine RE/ME | Urine protein creatinine ratio (mg/mg) | 24-h urinary protein (g/day) | Total cholesterol (mg/dl) |
|---|---|---|---|---|---|---|
| On presentation | 1.06 | 0.9 | Protein 4+ | 21 | 23.87 | 324 |
| Month 1 | 1.02 | 1.0 | Protein 4+ | 22 | 21.2 | 342 |
| Month 2 | 1.04 | 0.8 | Protein 4+ | 22.4 | 20.6 | 321 |
| Month 3 | 1.01 | 0.9 | Protein 4+ | 18.8 | 18.2 | 264 |
| Month 4 | 1.0 | 0.9 | Protein 4+ | 19.2 | 20 | 282 |
| Month 5 | 1.02 | 0.94 | Protein 4+ | 21 | 18.6 | 326 |
| Month 6 | 1.1 | 1.02 | Protein 3+ | 18.82 | 22.4 | 292 |
| Month 7 | 1.08 | 0.9 | Protein 4+ | 16.62 | 17.8 | 282 |
| Month 8 | 1.06 | 0.84 | Protein 4+ | 17.92 | 19.32 | 264 |
| Month 9 | 1.08 | 1.2 | Protein 4+ | 14.34 | 20.46 | 268 |
| Month 10 | 1.12 | 1.1 | Protein 4+ | 18.64 | 21.8 | 276 |
| Month 11 | 1.09 | 1.21 | Protein 4+ | 19.43 | 20.72 | 302 |
Discussion
ChAdOx1 nCoV-19 Coronavirus vaccine (recombinant) is a recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 spike (S) glycoprotein and is produced in the genetically modified human embryonic kidney 293 cells.[2] The vaccination is prioritized in renal disease patients due to its safety profile and increased mortality from coronavirus disease (COVID-19).
There were few rare reports of the new onset as well as relapse of glomerular diseases temporally associated with immunization probably due to heightened off-target immune response. Minimal change disease (MCD) and crescentic glomerulonephritis (GN) have been reported in temporal association with influenza and pneumococcal immunizations.[3] The autoimmune response elicited due to COVID vaccines resulting in GN may be due to molecular mimicry of an antigen (spike protein) with host proteins in genetically susceptible individuals.[4] Although the vector varies in both mRNA and adenoviral COVID vaccines (lipid nanoparticles versus replication-deficient adenovirus), both have been associated with glomerular disease onset and common antigenic target SARS-CoV-2 spike protein.[5] In all the case reports available in the literature, there is only a temporal relationship between the onset of symptoms and vaccination, causality is unclear. It may also be possible that immunization did not trigger the onset of glomerular disease within all these patients. Out of 26 patients reported till June 2021 with features suggestive of glomerular disease within three weeks of COVID-19 vaccination, Bomback et al.[6] commented that only two cases (MCD relapse) occurred after the single-dose adenoviral vector vaccines. In a case series of 29 patients with glomerular diseases post vaccination by Caza et al., 28 patients had native kidney biopsy (all of which were de novo GN), and there was one case of recurrent disease in a transplant recipient identified on allograft biopsy. Of the 29 patients, 27 received mRNA vaccines (11 Moderna, 12 Pfizer-BioNTech, and 4 unknown), and only two received adenoviral vaccines (one Johnson & Johnson/Jensen and one AstraZeneca). There were no cases of FSGS in the case series by Caza et al.[5] and Bomback et al.[6] Published case reports of GN either de novo or relapse after ChAdOx1 nCoV-19 vaccine are tabulated in Table 2.[5789101112] Till now nine cases of GN has been reported after ChAdOx1 nCoV-19 vaccine: 5 MCD (3 de novo, 2 relapse), 3 ANCA vasculitis (1 fresh, 2 relapse case), and one case of non-necrotizing granuloma around vessels. It is very interesting to note that in all these cases of GN, either de novo or relapse, symptoms onset occurred after the first dose of AstraZeneca vaccine.
| Study | Caza et al.[5] | Morlidge et al.[9]2 cases | Gillion et al.[8] | Villa et al.[10] | Anupama et al.[7] | Leclerc et al.[11] | David et al.[12] | Present case report |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 23 | Case 1: 30 Case 2: 40 |
77 | 63 | 19 | 71 | Case1: 75 Case 2: 74 |
21 |
| Sex | M | Case 1: M Case 2: F |
M | M | F | M | M both | M |
| Symptoms onset post COVID vaccination | 2 weeks post first dose | Case 1: 2 days post first dose Case 2: 1 day post first dose |
4 weeks post first dose | 7 days post first dose | 8 days post first dose | 13 days post first dose | Case 1: 5 weeks post first dose Case 2: 2 weeks post first dose |
12 days post first dose |
| Presentation | NS, AKI | Foamy urine, relapse of MCD Case 1: Post steroid, tacrolimus, rituximab Case 2: Post steroid+tacrolimus |
AKI | Deranged renal function | NS | NS | Old case of Case 1: Renal limited MPA Case 2: No renal involvement prior Both presented with deranged renal function |
NS |
| Serum creatinine | 2.9 mg/dl | Case 1: 0.9 mg/dl, Case 2: 1.18 mg/dl | 2.7 mg/dl | 2.90 mg/dl | 1.09 mg/dl | Oligoanuric AKI requiring hemodialysis Serum creatinine: 10.6 mg/dl |
Peak creatinine Case 1: 7.5 mg/dl Case 2: 9.96 mg/dl |
1.02 mg/dl |
| Proteinuria | 14 g/day | Case 1: UPCR- 142 mg/mmol, Case 2: Urine protein 3+ | Normal | Urine protein 2+ | UPCR: 3.18 | UPCR: 2,312 mg/mmol | 23.87 g/day | |
| Hematuria | Present | No | No | Mild hematuria | No | 6-10 RBCs | Yes | No |
| ANA | Positive | Not done | Negative | Neg | - | - | Negative | |
| ANCA | Negative | Not done | Negative | p ANCA positive | - | p ANCA positive in both | Negative | |
| Renal biospy | MCD | Not done | Noncaseating, non-necrotizing granuloma around small vessels | Focal class of ANCA associated GN | MCD (mesangial proliferative variant) | MCD/AKI | Active crescentic pauci immune GN suggestive of relapse | FSGS (tip variant) |
| Maximum follow-up | 3 weeks | Case 1: 10 days post steroid Case 2: 2 weeks |
4 weeks post steroid | 6 weeks post steroid + cyclophosphamide | Not mentioned | 68 days, off hemodialysis after 38 days | Case 1: RRT+steroid+rituximab Case 2: Steroid+cyclophosphamide |
11 months |
| Response to treatment | Yes | Yes in both cases | Yes. Had also humoral response 8 weeks post vaccination | Yes | Yes | Yes | Case 1: Dialysis-dependent Case 2: Renal dysfunction persists |
No response noted till now to steroid/tacrolimus/rituximab. Humoral response to vaccination noted |
| Follow-up serum creatinine | 1.0 | Same as earlier | Normal creatinine | 2.08 mg/dl | Normal | 1.2 mg/dl | 3.4 mg/dl | 1.02 mg/dl |
| Follow-up proteinuria | UPCR: 0.07 | Normal | Normal | Not mentioned | Normal | UPCR: 28 mg/mmol | - | 20.7 g/day |
NS=Nephrotic syndrome, AKI=Acute kidney injury, UPCR=Urine protein creatinine ratio, MCD=Minimal change disease
FSGS is a heterogeneous disease due to multiple biological mechanisms resulting in a specific pattern of injury on kidney biopsy. In a case series of 13 patients who developed new or relapsing GN post mRNA COVID vaccination, most cases were IgA nephropathy. Only one relapse case of FSGS-T was noted 3 weeks post second dose of BNT162b2 (Pfizer) COVID vaccination.[13] Another case of FSGS was reported 5 days post the first dose of COVID-19 BNT Pfizer vaccination, presenting with new-onset nephrotic syndrome.[14] Both these cases of FSGS—one FSGS-T relapse and the other fresh onset—were post Pfizer vaccination. Also, both the cases responded to regular immunosuppression. The first case responded to only steroid, while the second case responded to steroid + tacrolimus. Our case has not responded to steroid + tacrolimus + rituximab till now. To our knowledge, no cases of FSGS-T have been reported after the ChAdOx1 nCoV-19 vaccination so far. The published FSGS case reports post any COVID vaccination are shown in Table 3. In the index patient, causality is based on compelling temporal association, although we cannot demonstrate a direct link with vaccination. We should be vigilant when evaluating patients post vaccination, especially when symptoms related to renal diseases are present.
| Study | Dormann et al.[14] | Klomjit et al.[13] | Present case report |
|---|---|---|---|
| Age/Sex | 20 years/F | 29 years/F | 21 years/M |
| Vaccine type | BNT162b2 vaccine (Pfizer) | BNT162b2 vaccine (Pfizer) | AstraZeneca COVID-19 vaccine |
| Disease onset | Edema about 5 days after the first vaccination | Relapse of nephrotic syndrome 3 weeks post the second dose of vaccination | 12 days after the first dose of vaccine |
| Presentation and laboratory features | New-onset NS, proteinuria: UPCR: 10.3 g/g, Alb 2: 120 mg/dl, Cr: 0.47 mg/dl, Chol: 566, LDL: 350, TG: 302 mg/dl, biopsy: FSGS | FSGS tip variant relapse. Was in remission for 24 months before relapse | New-onset NS, Urine protein 4+, 24-h urinary protein: 23.87 g, serum creatinine: 1.06 mg/dl, serum albumin: 0.9 g/dl, and total cholesterol: 324 mg/dl |
| 24-h urinary protein: 10 g/day, serum albumin: 2.2 g/dl. Serum creatinine normal (0.6-0.7 mg/dl) | Renal biospy: FSGS tip variant | ||
| Treatment and response | Prednisolone 60 mg/Taper, partial remission, after 10 days: proteinuria: UPCR 3.6 g/g, Alb 2 280 mg/dl; after 28 days: proteinuria: UPCR: 5.5 g/g; Alb 2: 340 mg/dl, Cr: normal, Chol: 450, TG: 230 mg/dl | High-dose steroid+tacrolimus 3.5 months of follow-up: 24-h urinary protein: 3.7 g/day, serum albumin: 3.2 g/dl (partial remission) | High-dose steroid×4 months, low-dose steroid+tacrolimus×7 months (continuing) |
| High-dose steroid×3 months, low-dose steroid + tacrolimus× 7 months (continuing) | |||
| Given Inj. rituximab 500 mg iv weekly× 4 doses (6 weeks back in the last week of November and first week of December 2021) |
UPCR=Urine protein creatinine ratio, FSGS=Focal segmental glomerulosclerosis, TG=Triglyceride
Effective immune response to the spike protein involves both B- and T-cells. T-cells are the most important mediators in relation to the glomerular disease after the COVID-19 vaccine. Systemic dysfunction of the T-cells promotes the production of a glomerular permeability factor that induces the fusion of foot processes resulting in proteinuria. Also, T-cells provoke swift production of cytokines as interferon-γ, tumor necrosis factor-α, and interleukin-2. Podocytes have cytokine receptors which after vaccination may influence the cytoskeleton of podocytes, change the podocyte integrity, and result in proteinuria.[34615] Patients may have subclinical immune dysregulation being unmasked by immunization due to a surge in cell-mediated or antibody-mediated immune responses. mRNA vaccine compared with other types of vaccine (inactivated virus) results in a more potent immune response and therefore associated with a higher rate of GN. It is also to be noted that this unwanted immune activation occurs only in a very small percentage of vaccinated patients.[3415]
The index patient has not shown any response to prescribed therapies even partially, which is contrary to the published literature so far regarding response to the treatment in case of post COVID vaccination GN. As detailed data on the individual response will emerge in future, these types of cases may become more common in future. The patient had attained humoral immunity and has adequate COVID antibody level post vaccination. We are also not sure about his next dose and preferred type of vaccination. In view of the administration of Inj. rituximab, it should be delayed by at least about 5–6 months.
Another important question that comes into mind is what should be done next if he will continue to have persistent steroid-resistant/CNI-resistant nephrotic syndrome? In view of persistent nephrotic syndrome features despite 7 months of tacrolimus therapy, it will be more prudent to stop tacrolimus now, considering the patient to be resistant to CNI treatment. There is no hard evidence to treat steroid-resistant primary FSGS patients resistant to CNIs. In such cases, other treatment options include mycophenolate mofetil, high dose dexamethasone and ACTH, or discontinuation of immunosuppression may be considered.
Conclusion
There are only a few published case reports of GN (de novo/relapse) post inactivated virus-based vaccine compared to theoretically more potent mRNA vaccine. Contrary to the published reports of GN post-COVID vaccination who had responded to regular immunosuppression, this case is resistant to immunosuppressive therapy so far. This is the first reported case of de novo FSGS post AstraZeneca COVID vaccination and the second de novo FSGS case post COVID vaccination.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 2021. Ministry of Health and Family Welfare. Guidelines for COVID-19 vaccination of children between 15-18 years and precaution dose to HCWs, FLWs & 60+ population with comorbidities. Government of India. Available from: https://www.mohfw. gov.in/pdf/Guidelines for COVID 19vaccination for children and precaution dose 27th Dec 2021.pdf
- 2021
- Vaccine-associated kidney diseases: A narrative review of the literature. In: Saudi J Kidney Dis Transpl. Vol 30. Mumbai, India: Medknow Publications Pvt. Ltd; 2019. p. :1002-9.
- [Google Scholar]
- Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586-94.
- [Google Scholar]
- Glomerular disease in temporal association with SARS-CoV-2 vaccination: A series of 29 cases. Kidney. 360;021;2:1770-80.
- [Google Scholar]
- De Novo and relapsing glomerular diseases after COVID-19 vaccination: What do we know so far? Am J Kidney Dis. 2021;78:477-80.
- [Google Scholar]
- Nephrotic syndrome following ChAdOx1 nCoV-19 vaccine against SARScoV-2. Kidney Int Rep. 2021;6:2248.
- [Google Scholar]
- Granulomatous vasculitis after the AstraZeneca anti-SARS-CoV-2 vaccine. Kidney Int. 2021;100:706-7.
- [Google Scholar]
- Relapse of minimal change disease following the AstraZeneca COVID19 vaccine. Kidney Int. 2021;100:459.
- [Google Scholar]
- A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: Casualty or causality? Kidney Int. 2021;100:937-8.
- [Google Scholar]
- Minimal change disease with severe acute kidney injury following the Oxford Astra Zeneca Vaccine: A case report. Am J Kidney Dis. 2021;78:607-10.
- [Google Scholar]
- Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: A case series of two patients. Nephrology. 2022;27:109-10.
- [Google Scholar]
- Nephrotic syndrome after vaccination against COVID-19: Three new cases from Germany. Dtsch Arztebl Int. 2021;118:662-3.
- [Google Scholar]
- Minimal change nephrotic syndrome in an 82 year old patient following a tetanus-diphteria-poliomyelitis vaccination. BMC Nephrol. 2009;10:21.
- [Google Scholar]







