Translate this page into:
Association of Acute Interstitial Nephritis with Nivolumab in Renal Cell Carcinoma: A Case Report
Address for correspondence: Dr. Befa Noto-Kadou-Kaza, Department of Nephrology, Pitie-Salpetriere, 47-80 Boulevard de l'Hôpital, 75013, Paris, France. E-mail: bfanotokadoukaza@yahoo.fr
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Recently, a number of innovative anticancer agents such us the programmed death 1 (PD-1) immune checkpoint inhibitors have been developed. Nevertheless, this type of immunotherapy may be associated with immune-related adverse events whose pathophysiology is considered similar to those found in autoimmune diseases such as nephritis. We report the case of a 71-year-old female with metastatic renal carcinoma who underwent nephrectomy. After three lines of other chemotherapies (VEGF and mTOR inhibitors), the patient was treated by nivolumab (3 mg/kg) for 4 months and developed acute kidney injury 16 weeks after initiating this immunotherapy. Kidney biopsy displayed a diffuse extensive interstitial inflammation associated with moderate interstitial edema. The discontinuation of nivolumab and the administration of prednisone (at 1 mg/kg and tapered over 3 months) was an effective treatment of the interstitial edema and led to the recovery of the kidney function.
Keywords
Acute kidney injury
immune-checkpoint blockade
interstitial nephritis
Introduction
Treatment of metastatic renal cell cancer (mRCC) is evolving. Historically, cytokine-based immunotherapy was the mainstay of treatment. However, the advent of other effective therapies has led to a substantial reduction in its use. In particular, vascular endothelial growth factor (VEGF) and molecular target of rapamycin (mTOR) pathway inhibitors use has shown to improve progression-free survival and overall survival with manageable toxicities. Despite these benefits, it has become evident that these agents do not sustain durable responses.[12] Recently, a number of innovative anticancer agents have been developed such as immune checkpoint inhibitors programmed death 1 (PD-1 inhibitor).[3456] On the other hand, this therapy is correlated to immune-related adverse events (irAEs) that occur in up to 60% of treated patients, usually of a mild-moderate grade. The pathophysiology of these irAEs is considered similar to those due to autoimmune diseases, wherein activated lymphocytes target self-antigens.[7]
We report the case of a patient with an mRCC treated with Nivolumab, a immune-checkpoint inhibitor, who developed acute kidney injury (AKI).
Patient and Observation
A 71-year-old Caucasian female with essential hypertension and diabetes mellitus without any micro- or macro-vascular complications was assessed in the nephrology department of the Pitie Salpetriere Hospital of Paris (France) because of AKI. The baseline serum creatinine was around 102 μmol/l (after nephrectomy), corresponding to a glomerular filtration rate (GFR) of 1min according to CKD EPI formula. The patient was initially diagnosed with mRCC in right kidney with liver, spleen and pulmonary metastasis. After nephrectomy, she was treated with sunitinib (VEGF inhibitor) as the first line of chemotherapy for 12 months, but the treatment was ineffective. She then received Everolimus (mTOR inhibitor) as the second line chemotherapy for one year. Since this second line treatment was also ineffective, chemotherapy was continued with Axitinib (VEGF inhibitor) as the third line treatment. After one year of treatment with Axitinib, no significant remission was obtained.
During the three lines of chemotherapy, renal function remained stable ranging between 83 and 116 μmol/l of serum creatinine (Scr) level. Treatment of mRCC was continued with a fourth line chemotherapy based on nivolumab (3 mg/kg) every two weeks. After 4 months of nivolumab immunotherapy, the patient was admitted to the hospital due to fever, and an inflammatory syndrome with 144 mg/l of serum C-Reactive Protein (CRP). Lungs scan showed pneumonia without bacteriological documentation, and the patient was treated with rovamycin, amoxicillin and clavulanic acid, leading to a good resolution marked by the regression of the inflammatory syndrome. After admission, the serum creatinine was increased at 270 μmol/l compared with the baseline (93 μmol/l) at 8 weeks, indicating AKI [Figure 1]. Urinalysis revealed 0.20 g/l of protein (albumin/creatinine ratio 0.03 g/g), without hematuria or leucocyturia. Besides amoxicillin and esomeprazole, there was no other recent history suggestive of exposure to nephrotoxic agents such as intravenous iodinated contrast agents, non-steroidal anti-inflammatory drugs or analgesics.

- Serum creatinine during nivolumab treatment, after stopping nivolumab and during treatment by prednisone
Ultrasound showed that the left kidney remained 13 cm without urinary tract obstruction. The transjugular kidney biopsy showed 9 glomeruli. Cellularity and architecture were normal, and no active inflammation of the tuft, necrotizing lesions or cellular crescents was observed. There was diffuse extensive interstitial inflammation associated with moderate interstitial edema [Figure 2]. The infiltrates were composed of lymphocytes and plasma cells. Giemsa staining revealed only exceptional polymorphonuclear eosinophils. Granuloma or granulomatous lesions were not noted. There were no significant abnormalities of tubules. Immunofluorescence staining revealed no significant immune deposits. We concluded a diagnosis of acute interstitial nephritis (AIN).
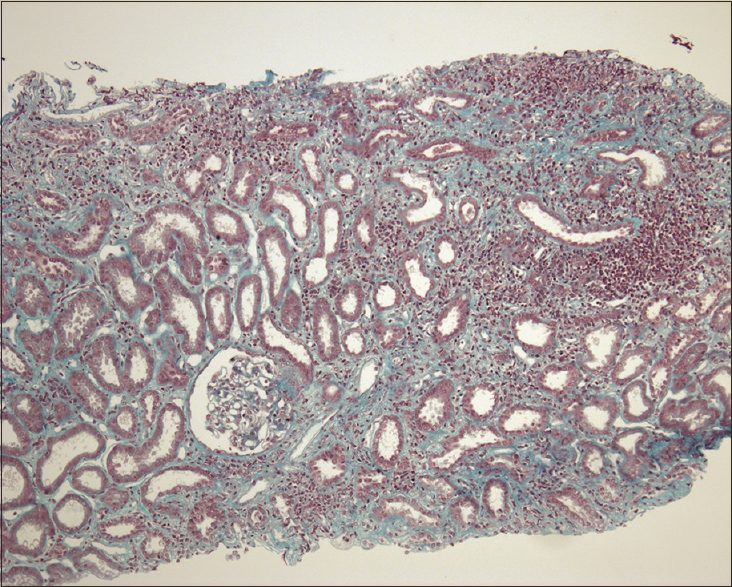
- Interstitial lymphocytic infiltrate, with tubular epithelial dystrophy, without glomerular abnormality. (Masson trichrome, magnification ×100)
Nivolumab therapy was withdrawn; prednisone therapy (1 mg/kg/d or 70 g/d) was initiated and tapered over 3 months, and the kidney function recovered to baseline.
Discussion
Immune checkpoint inhibitors exhibit immense tumor immunity via enhancing effector lymphocyte functions to target cancer cells by uncovering these molecular shields.[8] This immunotherapy mechanism is associated with an increased risk of irAE in melanoma and lung cancer patients; it has already been described.[9] However, severe renal AEs (grade 3–4) associated with a treatment based on nivolumab plus ipilimumab or nivolumab in monotherapy were still rare.[1011121314151617] To the best of our knowledge, there a limited number of recent cases of PD-1 inhibitor-associated AIN [Table 1],[18192021] and they are in patients with non–small cell lung cancer (NSCLC). In these studies, nivolumab was used in three cases, pembrolizumab in two other cases and bevacizumab in one case. In our case, we report a biopsy-proven AIN caused by nivolumab administered for mRCC treatment. Also, our patient displayed pneumonia, which was treated effectively by antibiotics and other AEs. The patient did not require dialysis but received steroid treatment. Kidney biopsy should be considered opportune in case of AKI to confirm AIN, even in a solitary kidney. Therefore, an incorrect diagnosis may lead to therapy discontinuation and a high-dose steroid treatment that could abrogate antitumor effects.
| Author | Number | Age/sex | Malignancy | PD1-inhibithor | Onset | Kidney biopsy | Treatement | Outcome |
|---|---|---|---|---|---|---|---|---|
| Shirali[18] | 6 | 73/M | NSCLC | Nivolumab | 11 months | Focal AIN | No | Resolved |
| 78/M | NSCLC | Nivolumab | 16 months | Diffuse AIN | Prednisone | Resolved | ||
| 60/F | NSCLC | Bevacizumab | 10 months | Focal AIN | Prednisone | Resolved | ||
| 69/F | NSCLC | Pembrolizumab | 3 months | Diffuse AIN | Prednisone | Resolved | ||
| 59/M | NSCLC | Nivolumab | 6 months | Focal AIN | Prednisone | Resolved | ||
| 69/F | NSCLC | Pembrolizumab | 12 months | Diffuse AIN | Prednisone | Resolved | ||
| Our case | 71/F | mRCC | Nivolumab | 4 months | Diffuse AIN | Prednisone | Resolved |
NSCLC: Non-small cell lung cancer, AIN: Acute interstitial nephritis, mRCC: Metastatic renal cell cancer
We acknowledge that our patient was receiving medications with known associations with AIN occurrence. For example, she was treated with proton pump inhibitors (PPIs) that may be associated with the idiosyncratic development of AIN in the long term[22]. However, our patients began the PPI therapy a long time before initiating the PD-1 inhibitor therapy. There was a delay between PD-1 inhibition and the development of AIN, similar to that reported for pneumonitis, which may occur after weeks or months after starting the PD-1 inhibitors therapy.[23] Although unclear, this finding suggests that the time from the PD-1 inhibitors therapy initiation to a break in tolerance is variable among the patients. In our case, AIN was observed four months after the start of the PD-1 inhibitor therapy. In the literature, time interval reported for this pathology is between 0.5 to 14.7 months.[18192021] AIN is an immuno-allergic phenomenon, which in general can occur at any time after the start of treatment. All patients are at risk for AIN in PD-1 inhibitors in general, but this risk is increased with preexisting renal dysfunction, dehydration or electrolyte disorders. To prevent AIN in PD-1 inhibitors therapy for all these patients, it's suggested to regularly monitor renal function to detect renal dysfunction early, which can facilitate to temporary cessation of treatment initiation of corticosteroids.[24]
We treated the patient with steroid according to the protocol based on a full dose (1 mg/kg/day) for 1 month followed by a gradual reduction over 3 months. After one month of steroid therapy, kidney function improved with more than 50% decline in serum creatinine Figure 1, suggesting the effectiveness of steroids therapy in PD-1 inhibitor-associated AIN.
Imputability score according to 2011 French reference is: C2S3I3 B0.[25]
Conclusion
AKI is a rare complication of PD-1 inhibitor therapy, which is manageable when AIN is confirmed. According to the literature and the results of our case, therapeutic approach to AIN included the discontinuation of the PD-1 inhibitor therapy and the administration of a variable course of steroids (generally, 1 mg/kg with a 1-month taper). PD-1 inhibitor therapy may be ideally avoided in case of AIN.
Further studies should be performed to evaluate the opportunity to continue treatment in combination with low-dose steroids. However, steroid therapy must be taken into consideration at the first sign of AKI without other identifiable cause.
Ethical approval
Ethical approval for this Case report was granted by the local Ethical Committee of Pitie Salpetriere Hospital of Paris (France).
Oral informed consent was obtained from the patient for publication.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207-25.
- [Google Scholar]
- Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J Biol Regul Homeost Agents. 2001;15:140-4.
- [Google Scholar]
- Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363:711-23.
- [Google Scholar]
- Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. NEJM. 2015;373:123-35.
- [Google Scholar]
- Pembrolizumab for the treatment of non-small-cell lung cancer. NEJM. 2015;372:2018-28.
- [Google Scholar]
- Nivolumab versus everolimus in advanced renal-cell carcinoma. NEJM. 2015;373:1803-13.
- [Google Scholar]
- Management of immune-related adverse events and kinetics of response with ipilimumab. JCO. 2012;30:2691-7.
- [Google Scholar]
- Co-inhibitory pathways and their importance in immune regulation. Transplantation. 2014;98:3-14.
- [Google Scholar]
- Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51-60.
- [Google Scholar]
- Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kid J. 2016;9:411-7.
- [Google Scholar]
- Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. JCO. 2006;24:2283-9.
- [Google Scholar]
- Ipilimumab-induced immune-related renal failure—a case report. Anticancer Res. 2012;32:4607-8.
- [Google Scholar]
- The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745.
- [Google Scholar]
- Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol Dial Transplant. 2019;34:108-17.
- [Google Scholar]
- Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287-91.
- [Google Scholar]
- Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor. J Immunother Cancer. 2017;5:3.
- [Google Scholar]
- Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638-47.
- [Google Scholar]
- Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer. 2016;115:1457-61.
- [Google Scholar]
- Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol. 2006;4:597-604.
- [Google Scholar]
- Causality assessment in pharmacovigilance: The French method and its successive udpates. Therapie. 2016;71:179-86.
- [Google Scholar]







