Translate this page into:
Bilateral Acute Renal Infarction Secondary to Methylene Tetrahydrofolate Reductase A1298C and PAI-1 Mutation
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We present this rare case of hyperhomocysteinemia due to a mutation in methylene-tetrahydrofolate-reductase (MTFHR) combined with plasminogen activator inhibitor deficiency, causing bilateral renal artery thrombosis. This case highlights the importance of genetic screening in individuals with a family history of thrombotic diseases. There seems to be a role of intervention, even in the setting of renal infarction.
Keywords
Acute renal infarction
hyperhomocysteinemia
intraarterial thrombolysis
Introduction
Renal artery thrombosis is a rare clinical entity. Most of the cases of renal artery thrombosis are a result of embolization due to cardiac event, trauma, dissection, and iatrogenic complication of endovascular procedures. Though renal artery thrombosis due to hypercoagulable state due to factor V Leiden mutation, antiphospholipid antibodies (APLA antibodies) and hyperhomocysteinemia can also occur. We report a case of renal artery thrombosis with renal infarction secondary to hyperhomocysteinemia in a young male.
Case Report
The index patient is a 23-year-old male who presented with complaints of fever, chills, left flank pain of 4 days duration. He did not give a history of dysuria, hematuria, cough, vomiting, loose stools, breathlessness, chest pain, joint pains, oral ulcers, or dryness of eyes. The patient was non-smoker and non-alcoholic. Clinical examination revealed a BP of 160/100 mm Hg and mild left lumbar tenderness. Computed tomography (CT) urography revealed absent contrast in the left urinary system, left renal artery thrombosis, and multiple renal infarcts [Figure 1]. The laboratory workup revealed, Hb-13 gm/dl, total leukocyte count (TLC)-13710/cmm, platelet-4,17000/cmm, creatinine - 1.46 mg/dL, blood urea nitrogen (BUN) -27.37 mg/dL, normal electrolytes and normal prothrombin time/international normalized ratio (PT/INR) and activated partial thromboplastin time (aPTT). The homocysteine levels were high (170 mmoL/L), serum antinuclear antibody (ANA) was negative. Patient was started on injection heparin (5000 IU bolus, followed by 1000 IU/hr). Diethylene-triamine-pentaacetate (DTPA) (Tc 99m) scan confirmed the absence of tracer uptake in the left kidney. Urine examination showed 2–3 rbc/hpf, no pus cells, and absent protein. Lipid profile showed elevated LDL (190 mg/dL), 2D ECHO was normal, and cardiac monitoring did not reveal arrhythmia. The serum C3 and C4 were normal. Protein C, protein S, anti-thrombin III were within normal range & factor V Leiden mutation, and APLA antibodies were negative. The fundus examination did not reveal retinal infarction. Computed tomography head did not reveal any area of infarction. Left nephrectomy was performed. In view of hyperhomocysteinemia, the patient was started on tab folic acid (10 mg/day). His serum creatinine reduced to 1.1 mg/dL, and he was discharged on warfarin and folic acid.

- CT angiography with pyelogram: Showing infarcted left kidney (blue arrow)
One week after getting discharged, the patient now developed right-sided flank pain associated with vomiting and decreased urine output, with no c/o fever, or chest pain. On physical examination, he had a BP of 154/100 mm Hg and a mild right lumbar and pelvic tenderness. Non-contrast CT abdomen did not show any significant abnormality. His creatinine increased to 3.2 mg/dL within 24 hrs. The INR was 2.1, and the DTPA scan now showed reduced tracer uptake in the right kidney. The ultrasound renal doppler showed resistive flow in the right renal segmental artery. The patient was restarted on anticoagulation (heparin 1000 IU/hr). His creatinine increased to 9.0 mg/dL, and he became anuric. He was started on hemodialysis and a repeat CT renal angiography [Figure 2] was suggestive of right renal segmental artery thrombosis with multiple renal infarcts. Urgent renal angiography and intra-arterial thrombolysis were done with alteplase, infused intravenously as an initial dose of 5 mg over 10 min, followed by 1 mg/hr for 36 hrs. He was continued on heparin (500 IU/hr) to avoid clotting in the arterial sheath. Check renal angiography revealed improved blood flow in the thrombosed vessel [Figure 3]. The patient required alternate day hemodialysis. Subsequently, in the next 2 weeks, the patient's urine output started improving gradually. After 3 weeks, the patient had 2.0 L/day of urine output, and his creatinine decreased to 2.8 mg/dL. In his family, his father had undergone coronary reperfusion (Percutaneous transluminal coronary angioplasty (PTCA)) at the age of 45 years. His grandfather and grandmother died due to myocardial infarction and stroke, respectively. On further investigations, the patient's thrombophilic profile revealed plasminogen activator inhibitor factor deficiency, and genetic mutation studies revealed a methylene-tetrahydrofolate reductase (MTHFR) mutation. These two defects predisposed him to a thrombotic state. His paternal aunt and uncle both were also found to have elevated homocysteine levels but no thrombotic event so far. The genetic analysis for hyperhomocysteinemia did not reveal any mutations in other family members. He was discharged on heparin (5000 IU twice per day), folic acid, aspirin, and statin. His serum creatinine came down to 2.4 mg/dL. It was proposed to start novel anticoagulants.
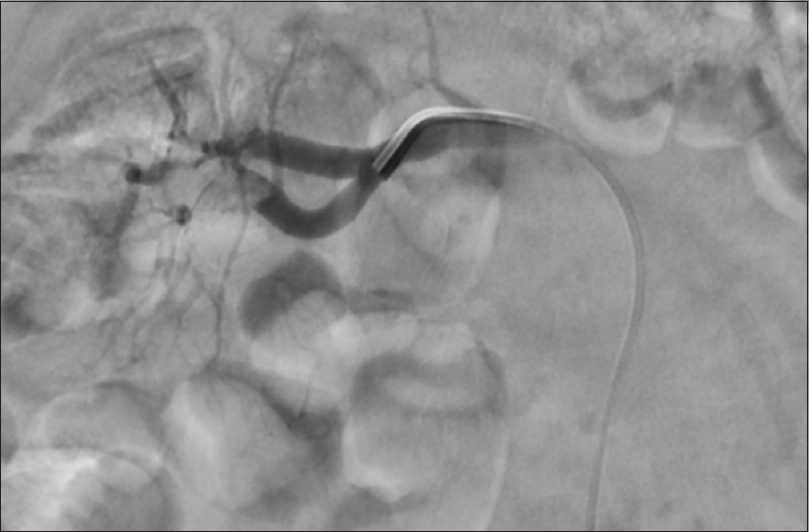
- Renal angiography before right renal artery thrombolysis
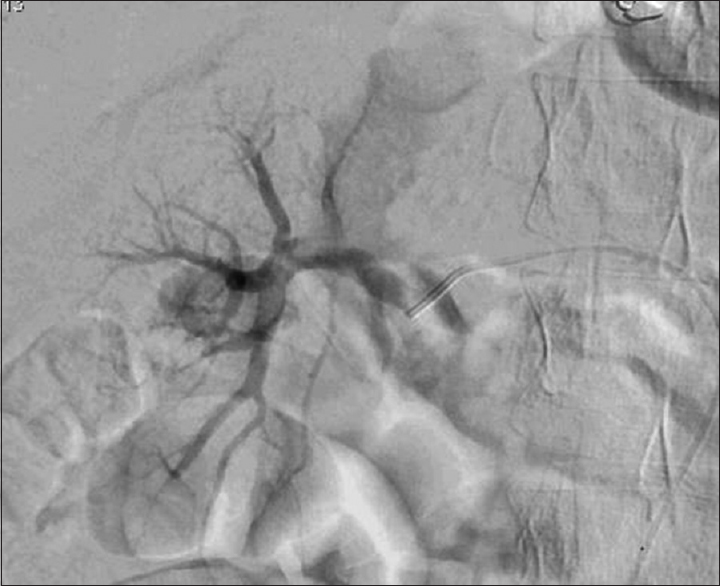
- Renal angiography after right renal artery thrombolysis
Discussion
Renal artery thrombosis is a rare clinical condition. The common causes of renal artery thrombosis are atrial fibrillation, renal artery trauma, endovascular revascularisation, infective endocarditis, and hypercoagulable states due to APLA antibodies, factor V Leiden mutation, protein C/S mutation and hyperhomocysteinemia. In a retrospective series of 38 patients together with a pooled analysis of 127 patients from separate previous reports (total of 165 patients), the most common cause for renal infarction was atrial fibrillation (37%) followed by coagulopathy and bacterial endocarditis.[1] In another multicentre retrospective study of 94 patients, which included patients between 1989 and 2011, the hypercoagulable group contained 15 patients with hereditary thrombophilia (16%, n = 6), hyperhomocysteinemia (n = 4), antiphospholipid syndrome (n = 4).[2] The diagnosis of renal artery thrombosis and renal infarction is usually not apparent because it can present as many other pathologic conditions like pyelonephritis, renal colic, acute abdomen, rupture of an aortic aneurysm etc. CT abdomen with intravenous contrast is the investigation of choice for renal artery thrombosis and infarction.[3] The gold standard investigation remains renal angiography, which has a benefit of intervention for treatment. Various epidemiological data have shown an increased risk for coronary artery disease, stroke, venous thromboembolism, and peripheral vascular disease in patients with elevated levels of homocysteine. Information from various randomized clinical trials has been unable to confirm convincingly that the lowering of homocysteine has improved clinical outcomes for the secondary prevention of cardiovascular disease.[4]
The optimal treatment for renal infarction due to thromboembolism is uncertain, given the absence of comparative studies. Open surgical revascularization has limited use due to significant morbidity and mortality. The endovascular options include intra-arterial fibrinolytic therapy, pharmaco mechanical thrombectomy, and aspiration thrombectomy with possible angioplasty, but successful results are anecdotally described.[5] Thus, the role of endovascular therapy in renal thromboembolism is yet to be determined. Anticoagulation is clearly indicated in cases of atrial fibrillation, left ventricular thrombus, or hypercoagulable conditions.[6]
As per our knowledge, this is the first case of renal infarction in the context of heterozygous MTHFR A1298C mutation in combination with hyperhomocysteinemia and positivity of plasminogen activator inhibitor (PAI) antibodies. These two conditions have been independently associated with an increased risk of thromboembolism. A homozygous MTHFR C677T with low-folate status was previously reported in a patient with renal infarction.[7] Interestingly heterozygous mutation involving a single nucleotide C677T polymorphism in the MTHFR gene leading to renal artery thrombosis has also been described, again associated with low folate and elevated homocysteine level. There seems to be a folate-dependent link between homozygous MTHFR mutation, the elevation of homocysteine levels and both arterial and venous thrombotic events.[8] The MTHFR 677TT genotype can be considered an idependent risk factor for both arterial and venous thrombosis in selected cases of patients without coexisting prothrombotic defects. Nevertheless, folic acid treatment for lowering homocysteine levels cannot be supported, given large meta-analysis data showing a lack of protective role in terms of cardiovascular events in patients with chronic kidney disease.[9]
The possibility of a synergistic relationship between the different hereditary thrombophilic states in our case cannot be excluded. The low number of patients with a combined coagulation defect, together with the rarity of an arterial thrombotic event, implies that conclusions cannot be made about the absolute level of thrombosis risk in combined hereditary thrombophilia. Our case demonstrates the complexity of a hypercoagulable state with both inherited (MTHFR, PAI antibodies) and acquired risk factors interacting to produce the thrombotic phenotype evidenced by renal artery thrombosis in both the renal arteries. Although the patient's father's genetic analysis was negative for MTHFR, his plasma homocysteine level was high (88 μmol/L). Similarly, his father's sister had a homocysteine level of 98 μmol/L, albeit a negative genetic screen, indicating a probability of genetic inheritance of hyperhomocysteinemia. The grandparents were not alive to undergo the genetic analysis.
Conclusion
The exact mechanism of homocysteine induced arterial thrombosis has been investigated and convincingly proven in several trials. However, there remains a paucity of data to explain this phenomenon associated with isolated renal artery thrombosis. Genetic testing should be done in patients with family history and no known comorbidities. The use of folic acid does not ensure the prevention of thrombosis in these patients. Renal reperfusion using thrombolysis despite renal infarction can be considered.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal infarction in the ED: 10-year experience and review of the literature. Am J Emerg Med. 2012;30:1055-60.
- [Google Scholar]
- Acute segmental renal infarction due to factor V Leiden. Arch Esp Urol. 2009;62:486-8.
- [Google Scholar]
- Clinical characteristics of patients with segmental renal infarction. Nephrology (Carlton). 2006;11:336-40.
- [Google Scholar]
- Erdogan Diagnosis and endovascular treatment of acute thromboembolic renal artery occlusion presenting with abdominal pain. J Thromb Thrombolysis. 2012;34:419-24.
- [Google Scholar]
- Hyperhomocysteinemia, low folate status, homozygous C677T mutation of the methylene tetrahydrofolate reductase and renal arterial thrombosis. Clin Nephrol. 2002;57:158-62.
- [Google Scholar]
- Low folate levels and thermolabile methylenetetrahydrofolate reductase as primary determinant of mild hyperhomocystinemia in normal and thromboembolic subjects. Arterioscler Thromb Vasc Biol. 1999;19:1761-7.
- [Google Scholar]
- C677T substitution in the methylenetetrahydrofolate reductase gene as a risk factor for venous thrombosis and arterial disease in selected patients. Haematologica. 1999;84:824-8.
- [Google Scholar]







