Translate this page into:
Carcinoma of the Tongue in Renal Transplant Recipients: An Unusual Spectrum of De novo Malignancy at a Tertiary Care Center in India Over a Period of 26 Years
Address for correspondence: Dr. R. Jha, Department of Nephrology, Virinchi Hospital, Road No. 1, Banjara Hills, Hyderabad - 500 034, Telangana, India. E-mail: jharatan08@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal transplant recipients are at a higher risk of malignancy. We report our experience and the critical differences in the presentation of malignancy in kidney transplant patients performed at our tertiary care center and followed up over the period of 1990–2015. A total of 338 live donor transplants performed in 332 patients were analyzed. Induction immunosuppression was used in 22 cases with interleukin-2 (IL-2) receptor antibody. Overall 299 patients were continued on calcineurin inhibitor (CNI)-based triple drug immunosuppression, 33 were off CNI with 13 of them receiving sirolimus additionally. A total of 16 malignancies including post transplant lymphoproliferative disease (5), oral cancer (5), lung cancer (2), hepatobiliary cancer (2), colon cancer (1), and skin cancer (1) were diagnosed in 15 patients. Over the 26-year follow up, 138 patients died of whom 12 died due to cancer. Cancer occurred in 4.7% of patients but accounted for 9.4% of deaths. Oral cancer occurred after a significantly longer latency of over 10 years (212 vs. 94 months, P = 0.00652). Despite the longer latency, oral cancer patients were younger at diagnosis (44.0 vs. 52 years, P = 0.01016) and had better outcome (Fisher's exact test, P = 0.0275). This was despite a longer overall follow-up for the oral cancer patients, reflecting the better outcome for these patients (24 vs. 4 months, P = 0.0278). This might be the result of relatively early diagnosis of oral cancers.
Keywords
Carcinoma tongue
malignancy
renal transplant
Introduction
Solid organ transplant patients are at an increased risk of malignancy as compared to general population (nearly 3–5 times) with serious consequences.[1] With significantly improved survival due to potent immunosuppressive drugs, better control of infectious complications, and cardiovascular events, there is an increase in incidence (average 5%–6%, range 1%–30%) of malignancies in these patients.[23] It is now emerging as one of the important causes of morbidity and mortality in a kidney transplant recipient (KTR) in the recent decade with functioning graft.[45] Their immunosuppressed state makes them more vulnerable to develop various malignancies, which also have wide geographical variation.[567] Predominant malignancies reported are epithelial (approximately 40%) and lymphoproliferative (approximately 20%). The common epithelial malignancies reported include non-melanoma skin cancer, lip cancer, carcinoma of genitourinary tract, and anorectal cancers while non-Hodgkin's lymphoma (post transplant lymphoproliferative diseases [PTLD]) is the other common malignancy.[289] Squamous cell carcinoma of the tongue is not commonly reported in KTRs with only isolated case reports published so far unlike lip, mouth, and tonsil cancers.[8] We present our retrospective analysis of single-center patients' data over 26 years with respect to incidence of malignancy, its spectrum, presentation, association, and survival.
Materials and Methods
A total of 338 live donor transplants were performed over the 26-year period (1990–2015) in 332 patients, with six patients having undergone second transplant. Details of malignancies developing in recipients and the outcome were analyzed retrospectively along with other causes of death such as infection, renal dysfunction, or cardiac events. Immunosuppression varied over the years. Initially, all received triple drug therapy. It consisted of prednisolone (332), azathioprine (AZA) (201), mycophenolate mofetil (MMF) (131), calcineurin inhibitor (CNI) either cyclosporine (260) or tacrolimus (72), and sirolimus in 13 patients. Induction immunosuppression was used in 22 cases with IL-2 receptor antibodies (basiliximab). Overall 299 patients were continued on conventional triple drug therapy. In 33 patients where graft biopsy showed changes of CNI toxicity, and in some with well-matched allograft, for economic reasons, CNI was gradually withdrawn and patients were maintained only on AZA/MMF with prednisolone, though 13 of them with no proteinuria received sirolimus additionally.
Appropriate evaluation was done to identify the malignant lesion and localize it. Histopathological examination including immunohistochemistry (Epstein–Barr virus/human papillomavirus [EBV/HPV]) was performed as deemed appropriate. Imaging including routine radiology, computed tomography scan, magnetic resonance imaging scan, and positron emission tomography scan was done depending on the need. Standard care of malignancy was given along with reduction of immunosuppression. The outcome was correlated with nature of immunosuppression, any induction, viral infections, renal dysfunction, rejection, or any known risk factors. A comparison was made between the nonoral malignancies (Group A) with oral cancers involving tongue (Group B) with respect to age, sex, time after transplant, duration of follow-up, and survival.
Statistical analysis
Median for all data was used instead of mean for statistical analysis. Nonparametric tests using median were used in view of the small numbers, outliers in data, and lack of normal distribution. Difference between patients characteristics were assessed with Fisher's exact test or Mann–Whitney test as appropriate. Fisher's exact test (two-tailed) with the P < 0.05 when comparing parametric data or Mann–Whitney test (two-tailed) with P < 0.05 for nonparametric data was considered statistically significant. Statistical analysis was done for both parametric and nonparametric data using an online calculator http://www.socscistatistics.com/tests/mannwhitney/default2.aspx accessed on 09 November 2016.
Results
Sixteen malignancies [Tables 1 and 2] were diagnosed in 15 patients (4.7%). Five patients had PTLD (33%) of which one developed it as second malignancy 5 years after initial hepatobiliary carcinoma. There were two cases of adenocarcinoma of the lung and another case of adenocarcinoma from sigmoid colon. The mean age of individuals developing malignancy at transplant was 38.4 ± 14.8 years (median 32) and at the time of malignancy was 51.5 ± 11.9 years (median 46). There were 138 deaths in the follow-up among 332 patients over 26 years with only 13 (9.4%) due to malignancy. The most common cause of death noted was infection in 54 persons (39%) followed by death arising from complications of renal allograft loss with uraemia 51 (36%) and cardiac 17 (12%).

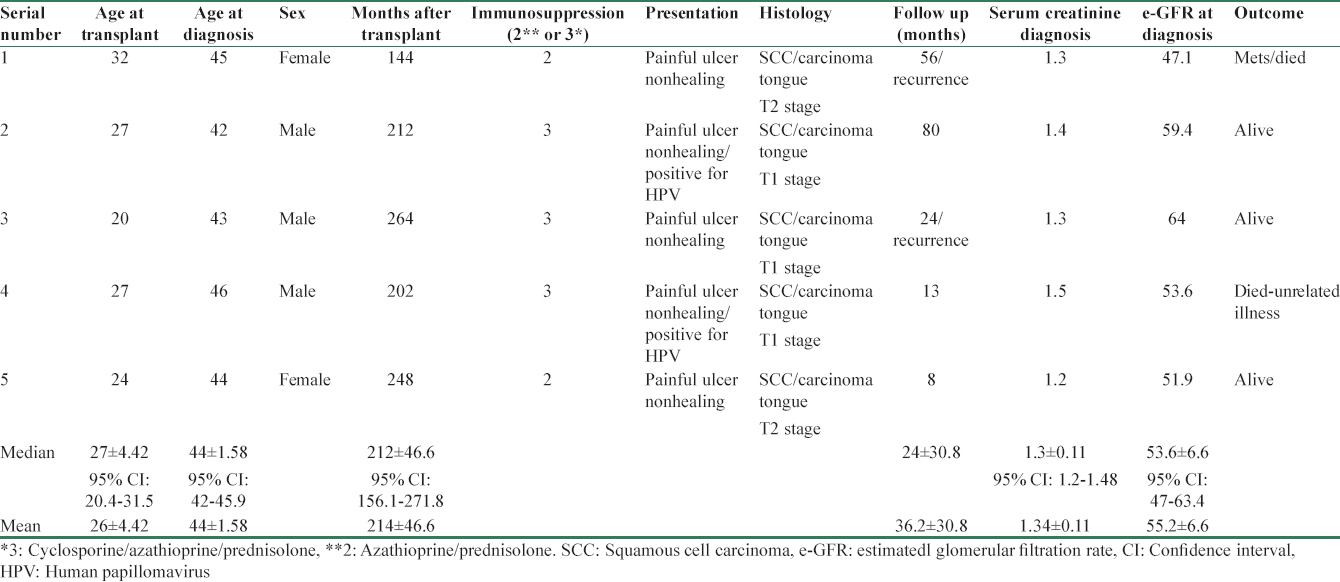
Demographic characteristics showed oral cancer developed in younger individuals (mean age at malignancy 44 ± 1.58 years/median 52) as compared to other group (mean age at the time of diagnosis of malignancy 53.8 ± 13.6/median 44). Patients in both Groups had reasonable and similar glomerular filtration rate (GFR) (median 54.5 vs. 53.6 ml/m). There was no significant difference in the number of rejection episodes and immunosuppressive therapy in both groups. All patients developing malignancy were getting prednisolone, AZA with or without cyclosporine as indicated in Tables 1 and 2 with none on tacrolimus or MMF. None of the patients who developed malignancy had received any depleting agent in our study.
All PTLD patients [Table 1] presented with either constitutional symptoms, fever, weight loss, or local organ-specific symptoms such as proptosis, dyspnea, and acute pain abdomen with perforation. All were late-onset PTLD. They were mostly located to gastrointestinal tract (GIT) (3/5) and extranodal (4/5). All displayed morphologically monomorphic population of large atypical lymphoid cells with CD20 positive marker with negative EBV status where tested (2/5) in tissue sample by in situ hybridization. Patients with adenocarcinoma of the lung or gut had local organ-specific symptoms [Table 1]. Patient with carcinoma of the skin had multiple warts such as lesions on legs with local lymphadenopathy. All patients in group A were treated with appropriate chemotherapeutic regimens along with reduction of immunosuppression (cyclosporine withdrawn and AZA reduced by 50%).
Five patients (33%) developed squamous cell carcinoma of the tongue [Table 2]. All the patients were in fifth decade (mean age 44 ± 1.58 years/median 44 years) and were on minimal immunosuppression. Three patients were of triple drug regimen (cyclosporine + AZA + prednisolone) and two patients only on AZA and prednisolone. They presented with nonhealing ulcer over tongue with no regional lymph nodes or distant metastasis. All five patients were maintaining fairly good graft functions with their mean serum creatinine of 1.3 mg% (median 1.3 mg/dl) (estimated GFR mean 55/median 54.5 ml/min). There were no obvious risk factors such as sharp tooth, tobacco or “pan-chewing” (betel leaf), and alcohol intake. All patients were treated with reduction of immunosuppression (cyclosporine withdrawn and AZA reduced by 50%). They underwent hemiglossectomy or wide local excision of the tumor along with regional lymph node resection. On histology, the resected margins were free from tumor, and in only one case, there was perineural and lymphovascular involvement. In all the cases, the resected lymph nodes were reported free from malignancy. Staging of T1N0M0 was made in four cases and one had T2N0M0. Immunohistochemistry for P16 antigen for HPV infection was done in four patients and it was positive in two. Follow-up radiotherapy was given in three cases along with adjuvant appropriate chemotherapy. Two cases had recurrence of growth at 2 and 4½ years after initial surgery needing second time local excision with radiotherapy and chemo-therapy. One patient died at 56 months with distant metastasis after her initial diagnosis and another at 13 months due to other coincidental illness. Three patients were still under follow up at 8, 24, and 80 months, respectively, after diagnosis.
Statistical analysis showed oral cancer patients were significantly younger at the time of diagnosis (44.0 vs. 52 years, Z-score 2.572, P = 0.01016). They developed it after longer latency (212 months vs. 94 months, Z-score −2.7189, P = 0.00652). Oral cancer patients had a longer follow-up (24 months vs. 4 months, Z-score −2.2045, P = 0.0278) and had better survival after diagnosis and treatment [Figure 1] compared to other cancer group with 3 out of five patients alive in oral cancer group at last follow-up as compared to 100% mortality in all the other non-oral cancer patients (Fisher's exact test P = 0.0275).
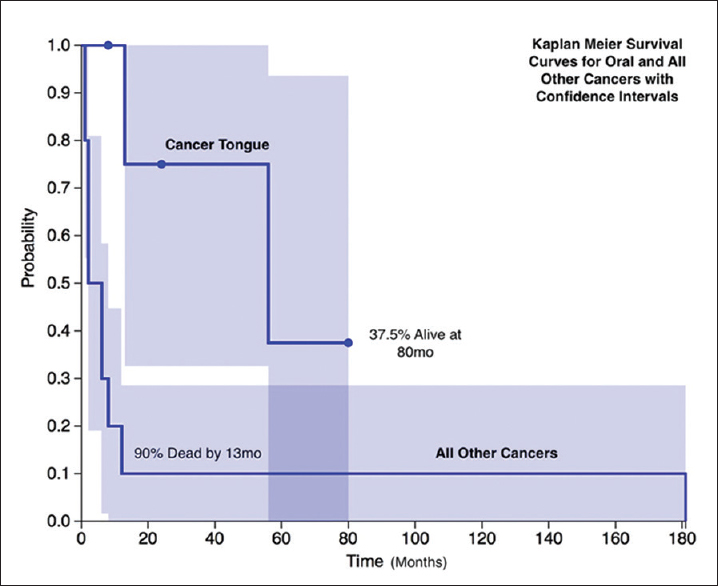
- Kaplan–Meier survival curves for oral and all other cancers with confidence intervals
Discussion
Early diagnosis of post transplant malignancies is an important and emerging challenge in the field of transplantation medicine and an even greater challenge is the prevention and management of malignancies. Long-term outcome of renal transplant recipients is improving due to better management of infections, cardiovascular complications, and immunological problems all over the world. At the same time, malignancy has emerged as an important cause for long-term morbidity and mortality.[45] Currently, malignancy is an important factor limiting life expectancy of transplant recipients with functioning allograft in the developed countries second only to cardiovascular events.[4569] However, scenario in South Asian region and a developing country like India is quite different where infections followed by chronic allograft loss from immunological and other causes are responsible for vast majority of death in the post transplant period. Cardiovascular complications and malignancy rank as other two less important causes of death.[10111213]
Compared to the risk of malignancy in the aging general population, KTRs have an earlier, higher, distinctive variable risk of usual and unusual malignancy.[1415] The chronic use of immunosuppressive agents to prevent allograft rejection increases the long-term risk of wide range of malignancy in solid organ transplantation depending on the nature of organ-transplanted and geographical location. The reported incidence of cancer in transplant cases varies from 1% to 30% in literature with recent data on occurrence of cancer after transplant are mainly derived from Israel Penn International Transplant Tumor Registry and Australia New Zealand Therapeutic Products Authority registry.[6161718] Recently, standardized incidence ratio (SIR) in various registries was calculated by comparing risk of malignancy among KTR to risk among general population matched for age, sex, year of diagnosis, which gives more meaningful reliable estimate than nonstandardized percent incidence[61416] and has characterized risk in KTR as high/medium/low risk based on SIR (>5, 1–5, <1). Overall, the risk of malignancy in KTR is 3–5 times than general population.[1]
Cancers in KTR could be characterized as de novo, preexisting or rarely donor derived due to unintended transmission of malignant cells from a donor that may result in metastasis. The risk of inadvertent transplantation of malignant cells appears to depend on the type and extent of the donor's cancer and is rare in live renal transplantation. Several factors have been linked to the increased incidence of malignancies among transplant recipients including age of transplant, genetic diversity, environment factors such as sun exposure, extent and duration of immunosuppression, concomitant viral infection, and duration of pretransplantation dialysis.[19] One of the important reasons for differential increase is due to increased propensity for various viral infections such as hepatitis B virus (HBV), hepatitis C virus, HPV, EBV, human herpes virus (HHV), cytomegalovirus, and Merkel cell polyomavirus (MCV) which are well known carcinogenic viruses in general population.[12021] Other microbial agents such as Helicobacter pylori have also been implicated in lymphoma associated with gastric mucosa.
Certain cancers have high incidence in KTR across the world such as skin cancer (non-melanoma), lip cancer, non-Hodgkin's lymphoma, Kaposi's sarcoma (KS), and genital and anorectal cancers unlike low incidence breast and prostate cancers.[151920] Although risk of oral cancers especially of lip have been reported to be increased in many such studies, carcinoma tongue has not been reported to be so. There seems to be some geographical variability in malignancy among renal transplant recipient with most common cancer being reported as skin cancer from Australia, Germany, and Hong Kong, while lymphoma from UK, Sweden, Germany, South Africa, India, and Pakistan.[61617182223] Increased incidence of KS from Saudi Arabia and gastrointestinal cancer from Japan has been reported.[24] However, there is no mention of increased risk of oral cancer involving tongue in any of such reports even from the South Asian region unlike the present study where carcinoma tongue was as common as PTLD. Most of the centers from India have reported high incidence of PTLD with low incidence of skin cancers.[222526] There have been only isolated reports of carcinoma tongue in various other studies and single case reports have been published from India.[222728]
Collett et al., in their study of 25,100 KTR of a large UK Registry data, reported only 34 cases of oral cancers that included palate, gum, floor of the mouth, tongue, and other part of mouth.[18] In Australia and New Zealand Dialysis and Transplant (ANZDATA) 2013 data of 25,700 patients, oral cancer was reported in 126 patients (4.6%) but no separate data were mentioned for carcinoma tongue.[16]
In contrast to most of the data reported from various regions in India, the present study has incidence of 4.7% that is 2–3 times higher but still less than that reported in transplant registries from other developed countries.[2223] Our study's overall low incidence of malignancy could be due to lower age of recipient at time of renal transplant, lower immunosuppression, no use of antithymocyte globulin, except for resistant rejection, missing follow-up data, relatively lower post renal transplant survival, and inadequate follow-up. There is a striking paucity of skin cancer though equally impressive is the presence of tongue cancer and late-onset PTLD with negative EBV status unlike reports from other centers of India and developed countries.
Although higher immunosuppression due to acute rejection is considered as risk factor for having malignancy in predisposed individuals, absence of any rejection could also mean a more immunosuppressed state, suggesting the need to reduce the immunosuppression further down to a minimum. This may be difficult to decide during routine clinical follow-up. The concept of scaling down immunosuppression to the lowest level should be aimed in such individuals who are at low immunological risk with stable renal function and no rejections. Use of mechanistic target of rapamycin (m-TOR) inhibitors may reduce the risk of malignancies in such recipients.[19] None of our patients who were on m-TOR inhibitors had malignancy.
At least four viruses may be considered co-carcinogenic in transplanted patients, including EBV, HHV-8, HPV, and MCV. We had noted this association in four of our patients (two patients with HBV and two with HPV). Two with carcinoma tongue had HPV co infection, one patient with hepatocellular carcinoma, and another with PTLD involving liver had chronic HBV infection. Known viral association of EBV infection was not seen in any of our patients of PTLD where it was checked. The cause for such high incidence of carcinoma tongue in the present study is not obvious. All the patients were relatively young (40–45 years) at the time of diagnosis. Many patients with oropharyngeal squamous cell carcinomas, particularly those arising in the base of the tongue and in the tonsillar region, do not have any of the traditional risk factors associated with head and neck cancers (e.g., smoking, smokeless tobacco, and alcohol consumption). Epidemiologic and molecular studies have identified the HPV-16 genotype of HPV as a causative agent in many of these patients.[21] In the present study, staining for P16 antigen for HPV infection was positive in two of the four specimens. None of these five patients had received induction therapy with lymphocyte depleting agents or IL-2 receptor blockers. All of them were on minimal immunosuppression. There was no correlation between any immunosuppression drugs to the occurrence of a specific cancer in follow-up population. Such high incidence of oral cancer was restricted geographically to a particular local population of southern part of India (Telangana and Andhra Pradesh) and only isolated cases are reported from other parts of India. We speculate therefore the role of diet in its causation though viral infection like HPV could be one such additional risk. The diagnosis of malignancy was after long duration of 12–22 years after transplantation. Even in other case reports from India, carcinoma tongue was diagnosed 9 and 11 years after transplant.[2829] This suggests that this malignancy is possibly more common in long-term survivors who have been immunosuppressed for a long period.
Cancers if diagnosed early continue to have better outcome as seen in oral cancer, which is a surface lesion. The diagnosis was made relatively early in all the five tongue cancer patients staged as T1 or T2 with no nodal involvement or metastasis. The prognosis of carcinoma of tongue in this setting of renal transplant seems to be better compared to some other malignancies. Though two patients died, one nearly 5 years after initial diagnosis with metastasis and another after 13 months with an unrelated illness, three patients have remained in remission and are alive. Hence, high index of suspicion in any nonhealing ulcer over tongue in a transplant patient is required to establish early diagnosis and get the best outcome. Other cancers of gut, lung, and lymphoma where the diagnosis is usually late have dismal prognosis. Survival after post renal transplant malignancy is poorer compared to transplant alone or cancer alone as evident from ANZDATA registry data. Conversely, the same registry showed increased death risk as high as four-fold in patients above age of 30 years both in males and female as compared to general, transplant, or cancer population.[9]
The presentation of PTLD is a highly variable and has different localization pattern for kidneys, lung, liver, central nervous system, lymph nodes, GIT/disseminated status needing high index of suspicion. Most common presentation of PTLD was nonlocalizing fever, pain abdomen, weight loss or chronic diarrhea. Though presentation is usually chronic, occasionally, it presented with acute features of viscus perforation or obstructive jaundice needing emergency surgery when the real diagnosis is unfolded. Associated local symptoms (breathlessness, hemoptysis, skin lesions, malabsorption) gave the clue to the potential site of malignancy in some instances. Hence, a patient having fever, weight loss, malaise if unremitting needs to be evaluated for its presence if other etiologies such as viral infection and tuberculosis have been excluded.
Cancer in KTRs is an enigmatic issue with limited choice of immunosuppression.[9] The ability to prevent and detect solid organ malignancies in the transplant patient, particularly early-stage carcinomas, relies on periodic screening and strict adherence to prophylactic measures.
Information about screening, treatment, monitoring strategies for such high-risk population is limited and largely extrapolated from information in general population. However, various published guidelines recommend for cancer screening periodically in KTR, which needs to be adhered to. The approach to post transplant malignancies begins with adopting general preventive measures such as avoiding excess immunosuppression or repeated exposure to antilymphocyte drugs, preferring immunosuppressive drugs, such as m-TOR inhibitors and IL-2 receptor antibodies to probably reduce the risk. Measures such as reduction or cessation of immunosuppressive therapy may result in tumor regression in lymphoma and skin cancers. In KS, reducing the CNI exposure may be particularly important. Periodic focused oral health and hygiene check and screening may be useful in addition to conventional screening for other malignancies such as cervix, gut, and lung.[9143031]
The major limitations of this study include the retrospective nature of the analysis, lack of information on tissue markers of EBV/HPV infection uniformly, and heterogeneity of immunosuppression used since these patients were transplanted at different times over three decades.
Conclusion
In our experience, PTLD and carcinoma of the tongue were the most common malignancies seen in kidney transplant recipients. While PTLD was seen usually early (within first decade of transplant) and was associated with dismal prognosis, carcinoma tongue was seen much beyond first decade with less aggressive course. Patients with oral cancer had a better outcome and are more likely to survive for longer periods of time following their diagnosis. We suggest that oral cancers could be related to dietary factors because it was seen in local population only and none in the non-local transplant. Viral infection could be a contributor in some patients and the role of vaccination needs exploration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank our colleague Lt. Col. (Dr.) J. Muthukrishnan, Classified Specialist (Med and Endocrinology), Southern Command Hospital, Pune, for helping statistical analysis and manuscript preparation.
References
- Cancer incidence after immunosuppressive treatment following kidney transplantation. Crit Rev Oncol Hematol. 2005;56:71-85.
- [Google Scholar]
- Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-901.
- [Google Scholar]
- Malignancy after renal transplantation: Incidence and role of type of immunosuppression. Ann Surg Oncol. 2002;9:785-8.
- [Google Scholar]
- Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation. 2011;91:225-30.
- [Google Scholar]
- 'United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59 1 Suppl 1:A7, e1-420.
- [Google Scholar]
- The Israel Penn International Transplant Tumor Registry. AMIA Annu Symp Proc 2003:1053.
- [Google Scholar]
- Racial and ethnic variations in incidence and pattern of malignancies after kidney transplantation. Medicine (Baltimore). 2005;84:12-22.
- [Google Scholar]
- Cancer after kidney transplant. In: Turner N, Lamier N, Goldsmith DJ, Winearls CG, Himmelfarb J, Remuzzi G, eds. Oxford Textbook of Clinical Nephrology Vol 3. (4th ed). Oxford, UK: Oxford University Press; 2016. p. :2483-90.
- [Google Scholar]
- Infections after renal transplantation in India. J Nephrol Ren Transplant [Review]. 2009;2:71-88.
- [Google Scholar]
- Causes of death in renal transplant recipients with functioning allograft. Indian J Nephrol. 2012;22:264-8.
- [Google Scholar]
- Post-transplant infections: An ounce of prevention. Indian J Nephrol. 2010;20:171-8.
- [Google Scholar]
- Infections after kidney transplantation: The bug bear of kidney transplantation in tropics. Open Urol Nephrol J. 2015;8:76-87.
- [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299-311.
- [Google Scholar]
- Cancer risk in patients receiving renal replacement therapy: A meta-analysis of cohort studies. Mol Clin Oncol. 2016;5:315-25.
- [Google Scholar]
- Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry Report 2013, 36th Report. Available from: http://www.anzdata.org.au/anzdata/AnzdataReport/36thReport/ANZDATA_36th_Annual%20_Report.pdf
- [Google Scholar]
- Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355:1886-7.
- [Google Scholar]
- Comparison of the incidence of malignancy in recipients of different types of organ: A UK Registry audit. Am J Transplant. 2010;10:1889-96.
- [Google Scholar]
- The janus face of immunosuppression – de novo malignancy after renal transplantation: The experience of the Transplantation Center Munich. Kidney Int. 2007;71:1271-8.
- [Google Scholar]
- Cancer risk following organ transplantation: A nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221-7.
- [Google Scholar]
- p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930-8.
- [Google Scholar]
- Spectrum of lymphoproliferative disorders following renal transplantation in North India. Indian J Nephrol. 2013;23:287-91.
- [Google Scholar]
- Posttransplant malignancies in renal transplant recipients: 22-years experience from a single center in Pakistan. Asian Pac J Cancer Prev. 2012;13:575-8.
- [Google Scholar]
- Cancers after renal transplantation: Multicenter experience. Saudi J Kidney Dis Transpl. 2008;19:825-30.
- [Google Scholar]
- Lymphoproliferative disorders in renal transplant recipients: A single-centre experience. Natl Med J India. 2006;19:50-1.
- [Google Scholar]
- Post-transplant lymphoproliferative disorders after live donor renal transplantation. Clin Transplant. 2005;19:668-73.
- [Google Scholar]
- Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-13.
- [Google Scholar]
- Carcinoma of the tongue in a renal transplant recipient: A rare post-transplant malignancy. Saudi J Kidney Dis Transpl. 2015;26:103-6.
- [Google Scholar]
- Squamous cell carcinoma of tongue in a renal transplant recipient. Indian J Med Paediatr Oncol. 2009;30:136-7.
- [Google Scholar]
- Cancer and mortality in solid-organ transplantation: Preventable or inevitable? Am J Kidney Dis. 2016;68:839-42.
- [Google Scholar]
- Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11(Suppl 15):S1-86.
- [Google Scholar]







