Translate this page into:
Retrospective Analysis of Spectrum of Infections and Antibiotic Resistance Pattern in Chronic Kidney Disease Patients on Maintenance Hemodialysis in a Tertiary Care Centre in North India
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Hemodialysis patients are at risk of infections. This study examines the spectrum of infections and antibiotic resistance patterns.
Methods:
We retrospectively reviewed the records of 586 hemodialysis patients from May 2018 to April 2020 in a tertiary care hospital in North India.
Results:
The study identified 99 episodes of confirmed infections. Urinary tract infections were the most common type of infections (55.5%), followed by catheter-related bloodstream infections (CRBSI) (definitive 21.2%). Other infections were pneumonia (8.1%), tuberculosis (6.1%), skin and soft tissue infection (4.0%), dengue fever (3.03%), and empyema thoracis (1.0%). Overall, Escherichia coli (33.3%) was the most common organism isolated. The most frequent uropathogens recovered were Escherichia coli (54%). In confirmed CRBSI, P. aeruginosa (23.8%) and MSSA (23.8%) were the most common pathogen isolated. K. pneumonia (37.5%) was the most common pathogen in pneumonia. Uropathogens showed the highest resistance to fluoroquinolones (93.3%–100%). Pathogens isolated in CRBSI showed maximum resistance to ciprofloxacin (100%). In pneumonia, the highest resistance was seen to third-generation cephalosporins (75%–100%).
Conclusion:
Though the bacterial spectrum remains the same over time, antibiotic resistance is changing in uropathogens. There is a trend of predominance of Gram-negative bacterial infections in CRBSI. Tuberculosis incidence rate was much higher than the general population. There is a need for nationwide and worldwide continuous surveillance.
Keywords
Antibiotic resistance
catheter-related bloodstream infection
chronic kidney disease
CRBSI
hemodialysis
Introduction
Infection is the second leading cause of death in patients on hemodialysis (HD).[1] HD patients visit emergency medical services around six times more often than their age-matched general populations, and infection is the second most common reason for hospitalization in this population.[2,3] Chronic kidney disease patients on HD are vulnerable to not only catheter-related bloodstream infections (CRBSI) but also to other types of infection. This subgroup of patients is at risk of infections due to malnourishment, impaired immunity secondary to renal failure, comorbidities, and breakdown of anatomical barriers due to repeated intravascular intervention for hemodialysis.[4-6] Though there are few recent Indian studies[7,8] on access-related infections, literature on non-access-related infections is scarce in HD populations. The aim of this study is to determine the spectrum of infections and the causative pathogens with their antibiotic resistance pattern in HD patients. Bacterial spectra and antibiotic susceptibility patterns vary geographically. The knowledge of infection epidemiology and antibiotic resistance patterns is necessary to choose optimal empirical treatment in such patients.
Material and Methods
Study design
Retrospective study of patients undergoing hemodialysis at the Department of Nephrology, AIIMS Jodhpur, Rajasthan, India over a 2-year period (May 2018–April 2020).
Eligibility criteria
Eligible subjects were patients on hemodialysis who had symptomatic, microbiologically confirmed infection. We excluded patients less than 18 years. For CRBSI definition (definitive and probable), the KDOQI guideline was used.[9] For urinary tract infection (UTI) case definition, symptomatic with urine culture showing growth of ≥105 colony-forming units/mL was used. For pneumonia, clinico-radiological evidence with isolation of infective organism from sputum or bronchial aspirate was used.
Data collection
Hospital electronic data records including patient discharge summaries were accessed for data collection. Culture and sensitivity reports of blood, urine, and other normally sterile body fluids were collected. Approval from the institute’s ethical committee was obtained.
Statistical analysis
Incidence of various types of infection rates was calculated. Definitive CRBSI rate was calculated per 1000 catheter days of hemodialysis. The number of catheter days was obtained by multiplying the average number of patients with catheters undergoing dialysis every year in the unit with the number of days in that calendar year. Incidence rate of pneumonia was expressed per 100 patient-years. Tuberculosis incidence was expressed per 100,000 patients per year. Descriptive statistics were used for data analysis, and the results were expressed in frequency or percentage. Statistical analysis was done using SPSS version 25 (Statistical Packages for the Social Sciences, Chicago, IL).
Results
Patient characteristics
The medical records of 586 patients requiring long-term hemodialysis between May 2018 and April 2020 were reviewed. There were 4967 dialysis days and 109,294 catheter days. In total, 99 microbiologically confirmed infective episodes occurred. The infective episodes occurred in 77 male (77.8%) and 22 female (22.2%) patients. Their median age was 40 years (20–80). Ninety-three (93.9%) out of 99 patients had only one infective episode, five (5.05%) had two, and one (1.01%) had three infective episodes.
Clinical infections
A total of 99 infective episodes were identified. Fever, chills, breathlessness, cough, urinary tract symptoms, and hypotension were the common presenting symptoms. UTIs were the most common type of infections (55.5%, n = 50). CRBSI was the second most common type of infection (confirmed: 21.2%, n = 21; probable: 6.1%, n = 6), followed by community acquired pneumonia (8.1%, n = 8). Tuberculosis (TB) was detected in 6.1% (n = 6), including four pulmonary TB, one bone TB, and one TB lymphadenitis. Other infections identified included skin and soft tissue infection (SSTI) in 4.04% (n = 4), dengue fever in 3.03% (n = 3), and empyema thoracis in 1.01% (n = 1).
Microbiology
Overall, Escherichia coli (33.3%) was the most common organism isolated, followed by Klebsiella pneumoniae and Pseudomonas aeruginosa (12.1% each). Gram-negative bacteria (GNB) were the most common isolate in UTI. The most frequent uropathogens recovered were Escherichia coli (54%, n = 27), K. pneumoniae (10%, n = 5), P. aeruginosa (10%, n = 5), and Enterobacter spp (10%, n = 5). In confirmed CRBSI, GNB accounts for 71.4% of the pathogen isolated, and the remaining 28.6% is made of up Gram-positive bacteria (GPB) such as Methicillin-sensitive Staphylococcus aureus (MSSA) and Enterococcus faecalis. Both P. aeruginosa (23.8%, n = 5) and MSSA (23.8%, n = 5) were the most common pathogen isolated. In community-acquired pneumonia, GNB were more common than GPB (87.5% vs. 12.5%), and K. pneumonia (37.5%, n = 3) was the most common respiratory pathogen, followed by Acinetobacter baumanii (25%, n = 2). Mycobacterium tuberculosis (MTB) was identified in six patients (6.1% of all the infections). SSTI was caused by MSSA (50%, n = 2), E. coli (25%, n = 1), and K. pneumonia (25%, n = 1). MSSA was isolated from one patient with empyema. Details of the microbiological profile are shown in Table 1.
| Pathogen | Types of infections | ||||
|---|---|---|---|---|---|
| UTI n (%) | CRBSI n (%) | Pneumonia n (%) | SSTI n (%) | Empyema n (%) | |
| Escherichia coli | 27 (54) | 3 (14.3) | - | 1 (25) | - |
| Klebsiella pneumoniae | 5 (10) | 3 (14.3) | 3 (37.5) | 1 (25) | - |
| Pseudomonas aeruginosa | 5 (10) | 5 (23.8) | 1 (12.5) | - | - |
| Enterobacter sp. | 5 (10) | 1 (4.8) | 1 (12.5) | - | - |
| Enterococcus faecalis | 4 (8) | 1 (4.8) | - | - | - |
| Burkholderia capacia | 2 (4) | 1 (4.8) | - | - | - |
| Acinetobacter baumanii | 1 (2) | 2 (9.5) | 2 (25) | - | - |
| CONS | 1 (2) | - | - | - | - |
| MRSA | - | - | 1 (12.5) | - | - |
| MSSA | - | 5 (23.8) | - | 2 (50) | 1 (100) |
CONS: Coagulase-negative staphylococcus, MRSA: Methicillin-resistant Staphylococcus aureus, MSSA: Methicillin-sensitive Staphylococcus aureus
Antibiotic susceptibility pattern
Uropathogens showed the highest resistance to fluoroquinolones (93.3%–100%), ceftriaxone (83.3%), and ampicillin (79.3%). Details of the antibiotic susceptibility in UTI are shown in Table 2 and Figure 1. Pathogens isolated in CRBSI showed maximum resistance to ciprofloxacin (100%), ceftriaxone (77.7%), and cotrimoxazole (66.6%), as shown in Table 3 and Figure 2. In pneumonia, the highest resistance was seen to third-generation cephalosporins (75%–100%), cotrimoxazole (75%), and fluoroquinolones (66.6%); the complete spectrum is given in Table 4 and Figure 3. In SSTI, MSSA showed resistance to penicillin G (50%) and full susceptibility to erythromycin, clindamycin, and levofloxacin; K. pneumonia and E. coli were resistant to all the antibiotics tested (penicillin G, piperacillin-tazobactam, ceftriaxone, cefepime, gentamicin, cotrimoxazole, and meropenem), with the exception of tigecycline and colistin. MSSA isolated from empyema did not show resistance to any of the drugs tested.
| Pathogens | Antibiotic resistance (% resistance) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP | PT | CT | CX | GN | AK | CP | LE | NX | CO | NT | MP | IP | EP | AZ | |
| E. coli† | 88.8 | 53.8 | 50 | 91.3 | 28 | 30 | 100 | 100 | 100 | 58.3 | 4 | 11.1 | 50 | 25 | 100 |
| K. pneumoniaeǂ | 100 | 50 | 100 | 80 | 50 | 66.6 | 50 | - | 100 | 80 | 60 | 66.6 | 100 | 100 | 100 |
| P. aeruginosa | - | - | 0 | 80 | 50 | - | 100 | 0 | 100 | - | - | 0 | 33.3 | - | 0 |
| Enterobacter | 100 | 0 | 0 | 0 | 66.6 | - | - | 0 | 100 | 0 | 100 | 0 | 0 | 0 | 0 |
| E. faecalis§ | 33.3 | - | - | - | 50 | - | 100 | - | - | - | 33.3 | - | - | - | - |
| B. capacia | - | 0 | 0 | - | - | 100 | 100 | 100 | - | 0 | 0 | 100 | - | - | - |
| A. baumanii | 0 | 0 | 0 | 0 | 0 | - | 100 | 0 | - | 100 | - | - | - | - | - |
| CONS¶ | - | - | 0 | - | 100 | - | 100 | - | - | 100 | - | - | - | - | - |
| Overall resistance | 79.3 | 26.4 | 23 | 83.3 | 51.4 | 31.3 | 93.3 | 93.3 | 100 | 58.8 | 20 | 25 | 37.5 | 28.5 | 25 |
AM: Ampicillin, PT: Piperacillin-tazobactam, CT: Ceftazidime-tazobactam, CX: Ceftriaxone, GN: Gentamicin, AK: Amikacin, CP: Ciprofloxacin, LE: Levofloxacin, NX: Norfloxacin, CO: Cotrimoxazole, NT: Nitrofurantoin, MP: Meropenem, IP: Imipenem, EP: Ertapenem, AZ: Aztreonam, † & ǂ: No resistance to Fosfomycin, § & ¶: No resistance to Vancomycin

- Overall antibiotic resistance pattern in urinary tract infection
| Pathogens | Antibiotic resistance (% resistance) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | CT | CX | CZ | GN | AK | CP | LE | CO | MP | IP | EP | AZ | TP | |
| S. aureus (MSSA)† | - | - | - | - | 0 | - | 100 | 100 | 80 | - | - | - | - | 66.6 |
| P. aeruginosa | 25 | 0 | - | - | 60 | 40 | 100 | 0 | - | 75 | 50 | - | 60 | - |
| K. pneumoniae | 33.3 | 100 | 66.6 | 33.3 | 50 | 50 | - | 66.6 | - | 33.3 | 0 | 50 | 50 | - |
| E. coli | 66.6 | 50 | 100 | - | 66.6 | 33.3 | 100 | - | 66.6 | 66.6 | - | 50 | 100 | - |
| A. baumaniiǂ | - | - | 100 | - | - | 50 | 100 | - | - | 50 | - | - | - | - |
| E. faecalis§ | - | - | - | - | 0 | 0 | - | 0 | - | - | - | - | - | 0 |
| B. capacia | - | 0 | - | - | 0 | - | - | 0 | 100 | 0 | - | - | - | - |
| Enterobacter | - | 0 | 0 | - | 0 | 0 | - | - | 100 | 0 | 0 | 0 | 0 | - |
| Overall resistance | 33.3 | 28.5 | 77.7 | 20 | 33.3 | 38.4 | 100 | 30.7 | 66.6 | 50 | 33.3 | 40 | 63.4 | 50 |
PT: Piperacillin-tazobactam, CT: Ceftazidime, CX: Ceftriaxone, CP: Cefoperazone, GN: Gentamicin, AK: Amikacin, CP: Ciprofloxacin, LE: Levofloxacin, CO: Cotrimoxazole, MP: Meropenem, IP: Imipenem, EP: Ertapenem, AZ: Aztreonam, TP: Teicoplanin, † & §: no resistance to Vancomycin and Linezolid, ǂ: No resistance to Colistin
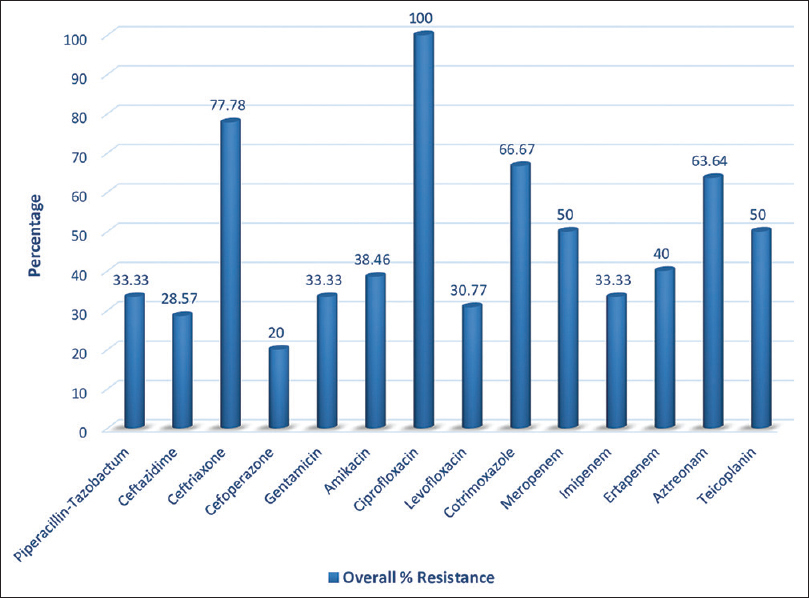
- Overall antibiotic resistance pattern in CRBSI
| Pathogens | Antibiotic resistance (% resistance) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | CT | CX | CF | CZ | GN | CP | LE | CO | MP | AZ | |
| S. aureus (MRSA) | - | - | - | - | - | 0 | - | 100 | 0 | - | - |
| P. aeruginosa | 0 | 0 | - | 0 | - | 0 | 0 | - | - | - | 0 |
| K. pneumoniae | 66.6 | 100 | 100 | 100 | 50 | 66.6 | 100 | 50 | 100 | 66.6 | 100 |
| A. baumanii | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - |
| Enterobacter | 0 | - | 100 | 100 | 0 | 0 | - | 0 | 0 | 0 | - |
| Overall resistance | 57.1 | 75 | 100 | 85.7 | 100 | 50 | 66.6 | 66.6 | 75 | 66.6 | 50 |
PT: Piperacillin-tazobactam, CT: Ceftazidime, CX: Ceftriaxone, CF: Cefepime, CZ: Cefoperazone, GN: Gentamicin, AK: Amikacin, CP: Ciprofloxacin, LE: Levofloxacin, CO: Cotrimoxazole, MP: Meropenem, AZ: Aztreonam

- Overall antibiotic resistance pattern in Pneumonia
Discussion
The study was done to retrospectively analyze the spectrum of confirmed infective episodes and antibiotic resistance patterns, and it was not designed to show risk factors or outcomes. Data were compiled from the institute’s hospital information system where all the patients’ clinical, radiological, and laboratory data are stored.
The study included 4,967 dialysis days, 109,294 catheter days, and there were 99 episodes of confirmed infection requiring therapeutic intervention. The overall infection rate was 19.9 episodes per 1000 days of dialysis, which was much higher than the 5.7 per 1000 days of dialysis reported by Berman et al.[10]
UTI in hemodialysis patients is less well studied, the literature is scarce, and the exact incidence remains unknown in this group of patients. D’Agata et al.[11] reported that UTIs were the most common nosocomial infections among chronic hemodialysis populations, accounting for 47% of all the infections. They also found that UTIs were more common among the chronic hemodialysis populations (4.2/1,000 patient-days) compared with patients not receiving hemodialysis (0.7/1,000 patient-days). Among hemodialysis patients, enterococci and Candida spp were the most common organisms in contrast to Staphylococcus aureus and coagulase-negative staphylococci among patients not on hemodialysis. They did not include community-acquired infections in the study. In our study, both communities acquired and hospital-acquired infections were included. UTIs were the most common infections, accounting for 55.5% of all the infections noted. In contrast to the previous study, Gram-negative bacteria were the most common pathogen (90% of all the UTIs, 54% of those E. coli). Considering frequent contamination or colonization, we excluded Candida spp isolated from urine specimens in our study. The bacterial spectrum causing UTIs remains the same compared to reports from the Indian general population.[12,13] There was high overall antibiotic resistance to ampicillin, ceftriaxone, fluoroquinolones, aminoglycosides, and cotrimoxazole. Compared to other Indian studies in general populations, we noted a higher level of overall antibiotic resistance to fluoroquinolones and carbapenems.[13] Considerable susceptibility still retained to piperacillin-tazobactam, ceftazidime-tazobactam, carbapenems, aztreonam, nitrofurantoin, and fosfomycin.
There has been a significant decline in the incidence of CRBSI over the past decade across the globe. During the study period, there were 21 definitive CRBSI episodes, of which 17 (80.95%) occurred in temporary catheters and four (19.05%) in permanent tunneled catheters, and six episodes of probable CRBSI. The overall incidence rate was 0.24 per 1000 catheter-days (definitive: 0.19/1000, probable: 0.054/1000), which is lower than that in older studies (5.3–6.5/1000 catheter-days).[14-16] However, our finding is comparable to a recent Indian study (0.2/1000 catheter-days) and a recent study from Alberta, Canada (0.19/1000 catheter-days).[7,17] While reports from developed countries consistently showed the predominance of Gram-positive bacteria (GPB),[18,19] recent reports from the developing world showed a trend of Gram-negative bacteria (GNB) predominance.[8,20] In our study, GNB accounts for 71.4% of the pathogen isolated, and the remaining 28.6% is made of up GPB such as S. aureus (MSSA) and E. faecalis. Both P. aeruginosa and S. aureus (MSSA) were the most common pathogen isolated (23.8% each), which is similar to Gupta et al.[8] report of P. aeruginosa predominance, and Shah et al.[7] report of S. aureus (MSSA) predominance. S. aureus showed high resistance to fluoroquinolones and cotrimoxazole, but no resistance to vancomycin or linezolid. P. aeruginosa showed high resistance to ciprofloxacin (100%), meropenem (75%), gentamicin (60%), and aztreonam (60%); lower resistance to piperacillin-tazobactam (25%) and amikacin (40%); and no resistance to levofloxacin. B. capacia and Enterobacter showed resistance only to cotrimoxazole. No drug resistance was noted in E. faecalis. Based on this study, it is still advisable to use empirical GPB and GNB coverage with vancomycin plus aminoglycoside for CRBSI episodes in this part of India. However, each institution should have its own data and recommendation.
It is surprising that pneumonia and the causative organisms in dialysis patients have received relatively less research attention. Estimated mortality rates from pneumonia in dialysis populations are 14–16 times more than in the general population.[21] Guo et al.[22] reported pneumonia incidence of 27.9/100 patient-years in the hemodialysis population. A subsequent observational study in the dialysis population found a pneumonia incidence of 21.4/100 patient-years, 30-day mortality of 10.7%, and that 90.1% required hospitalization.[23] Berman et al.[10] found that pneumonia contributed 13% of all infective episodes in patients on long-term dialysis. In our study, pneumonia contributed to 8.1% of the infective episodes, and the incidence of pneumonia was 0.68/100 patient-years, which is much lower than that noted in previous studies. The incidence of pneumonia per 1000 person-years in our study (13.6/1000 person-years) was comparable to the estimated incidence in the general population (1.5–14/1000 person-years).[24] The lower incidence of pneumonia in our analysis can be explained by the selection bias of our inclusion criteria which excluded many patients with discharge diagnosis of pneumonia, either due to nonavailability of culture report or negativity of culture of respiratory specimens. In end-stage kidney disease, Chen et al.[25] reported aerobic Gram-positive organisms’ predominance (54%, with 67% of those S. aureus). However, in a study among the hemodialysis population by Slinin et al.,[26] no pathogen was specified in 81.8% of cases, Gram-negative bacteria isolated in 11.1% cases (25% of those P. aeruginosa, the most common GNB), and 4.8% were attributed to Gram-positive bacteria. In our study, 87.5% (n = 7) cases were attributed to GNB, and GPB isolated in 12.5% (MSSA, n = 1) cases. K. pneumoniae was the most common organism (37.5%, n = 3), followed by A. baumanii (25%, n = 2). The organisms showed non-susceptibility to all the drugs tested.
TB remains an important cause of mortality and morbidity worldwide, especially in developing countries. Uremia is associated with impaired immunity due to various factors.[27,28] Meta-analysis of hospital cohorts and regional registries showed that dialysis populations have an increased risk for active TB compared with the general population (pooled unadjusted rate ratio of 7.7).[28] The same meta-analysis showed that after adjusting for demographic characteristics such as age and country of birth, the pooled rate ratio for TB risk decreased to 3.6 (95% confidence interval: 1.8–7.3). Country of birth was an important risk factor in dialysis populations rather than the dialysis state itself.[28] In our study, the incidence rate of TB was 1023.9 per 100,000 per year, which is 5.3 times higher than the general population in India (193/100,000 in the year 2019) but lower than that in an older report from Indian HD populations (4200/100,000 per year).[29,30] Incidence rates reported from other countries (5.7–115/100,000) are much lower than that in the Indian data.[27,31-35] Similar to a previous report from India, pulmonary TB was more common than extra-pulmonary TB (66.6% and 33.4%, respectively) in our study.[29] No drug-resistant TB was detected in the study.
Other less common infections noted in this study were SSTI, dengue fever, and isolation of MSSA from empyema in one patient. Isolates from SSTI include MSSA, multidrug-resistant K. pneumoniae, and E. coli. MSSA had good sensitivity to FQ, aminoglycosides, and clindamycin. Both K. pneumoniae and E. coli were resistant to penicillin, cephalosporins, aminoglycosides, and carbapenems.
The major limitation of the study is that it did not distinguish between community-acquired and hospital-acquired infections. There was no consideration of predisposing clinical conditions; thus, the reason for the very high incidence of UTIs could not be ascertained. Pneumonia incidence was exceptionally low because of selection of only microbiologically confirmed cases.
Conclusion
Our analysis provides a detailed spectrum of infections, pathogens, and their drug susceptibility in HD populations. The analysis included important non-access-related infections, which received less research attention from nephrologists. Though the bacterial spectrum remains the same over time, antibiotic resistance is ever-changing in UTIs. There is a trend of predominance of Gram-negative bacterial infections in CRBSI, but the commonly used empirical therapy with vancomycin plus aminoglycoside is still recommendable. Bacterial pneumonia and tuberculosis incidence were much higher than in the general population. There is a need for continuous surveillance for appropriate empirical antibiotic policymaking and to reduce the rising antibiotic resistance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758-64.
- [Google Scholar]
- Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26:3141-50.
- [Google Scholar]
- Emergency department use and hospital admissions among patients with end-stage renal disease in the United States. JAMA Intern Med. 2016;176:1563-5.
- [Google Scholar]
- EPIBACDIAL: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9:869-76.
- [Google Scholar]
- Septicemia in dialysis patients: Incidence, risk factors, and prognosis. Kidney Int. 1999;55:1081-90.
- [Google Scholar]
- Bacterial infections in hemodialysis patients: Pathogenesis and prevention. Kidney Int. 2005;67:2508-19.
- [Google Scholar]
- Incidence and etiology of hemodialysis catheter related blood stream infections at a tertiary care hospital in Mumbai: A 5 year review. Indian J Nephrol. 2020;30:132-3.
- [Google Scholar]
- Microbiology of non-tunnelled catheter-related infections. J Clin Diagn Res JCDR. 2016;10:DC24-8.
- [Google Scholar]
- National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy, and vascular access. Am J Kidney Dis. 2006;48:S1-322.
- [Google Scholar]
- Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis. 2004;39:1747-53.
- [Google Scholar]
- Hospital-acquired infections among chronic hemodialysis patients. Am J Kidney Dis. 2000;35:1083-8.
- [Google Scholar]
- Clinico-microbiological profile of urinary tract infection in south India. Indian J Nephrol. 2011;21:30-6.
- [Google Scholar]
- Retrospective analysis of antibiotic resistance pattern to urinary pathogens in a Tertiary Care Hospital in South India. J Basic Clin Pharm. 2014;5:105-8.
- [Google Scholar]
- Outcome and complications of temporary haemodialysis catheters. Nephrol Dial Transplant. 1999;14:1710-4.
- [Google Scholar]
- Bacteremia associated with tunneled, cuffed hemodialysis catheters. Am J Kidney Dis. 1999;34:1114-24.
- [Google Scholar]
- Haemodialysis catheter-related blood stream infection in ESRD patients: Incidence, outcome and antibiogram of the isolated organisms. Int J Adv Med. 2016;3:912-9.
- [Google Scholar]
- Catheter-related blood stream infections in hemodialysis patients: A prospective cohort study. BMC Nephrol. 2017;18:357.
- [Google Scholar]
- The spectrum of infections in catheter-dependent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2247-52.
- [Google Scholar]
- Determinants and outcomes of access-related blood-stream infections among Irish haemodialysis patients;A cohort study. BMC Nephrol. 2019;20:68.
- [Google Scholar]
- Pattern of causative micro-organsims in catheter related blood stream infections in dialysis patients: Experience from Saudi Arabia. J Ayub Med Coll Abbottabad. 2017;29:635-40.
- [Google Scholar]
- Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120:1883-7.
- [Google Scholar]
- Pneumonia in incident dialysis patients—The United States renal data system. Nephrol Dial Transplant. 2008;23:680-6.
- [Google Scholar]
- The clinical and economic burden of pneumonia in patients enrolled in Medicare receiving dialysis: A retrospective, observational cohort study. BMC Nephrol. 2016;17:199.
- [Google Scholar]
- Different bacteriology and prognosis of thoracic empyemas between patients with chronic and end-stage renal disease. Chest. 2007;132:532-9.
- [Google Scholar]
- Clinical epidemiology of pneumonia in hemodialysis patients: The USRDS waves 1, 3, and 4 study. Kidney Int. 2006;70:1135-41.
- [Google Scholar]
- Risk of tuberculosis in dialysis patients: A nationwide cohort study. PLoS One. 2011;6:e29563.
- [Google Scholar]
- Risk of active tuberculosis in chronic kidney disease: A systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:1493-9.
- [Google Scholar]
- Clinical profile of tuberculosis in patients with chronic kidney disease: A report from an endemic Country. Saudi J Kidney Dis Transplant. 2019;30:470-7.
- [Google Scholar]
- Global tuberculosis report 2020. Available from: https://www.who.int/publications-detail-redirect/9789240013131
- Risk of tuberculosis in dialysis patients: A population-based study. Int J Tuberc Lung Dis. 1998;2:989-91.
- [Google Scholar]
- Tuberculosis infection in Chinese patients undergoing continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2001;38:1055-60.
- [Google Scholar]
- Tuberculosis in hemodialysis patients in area of high incidence of mycobacterium tuberculosis and human immunodeficiency virus infection. Dial Transplant. 2008;37:486-90.
- [Google Scholar]







