Translate this page into:
Changing Tides of Acute Interstitial Nephritis: A Retrospective Observational Study from South India
Address for correspondence: Dr. Mythri Shankar, Nephrology, Institute of Nephro-Urology, Victoria Hospital Campus, KR Market, Bengaluru, Karnataka, India. E-mail: mythri.nish@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The incidence of acute interstitial nephritis (AIN) has been increasing in recent years. The causes and outcomes of AIN have been changing with time and vary widely based on geographical region.
Methods:
A retrospective observational study was conducted in a tertiary care center. All (n = 6234) native kidney biopsies were reviewed from January 2016 to December 2021. All biopsy-proven AIN cases were included in the study. AIN associated with systemic diseases (such as SLE, Sjogren’s, sarcoidosis, plasma cell dyscrasias), proliferative glomerulonephritis, and allograft biopsies were excluded.
Results:
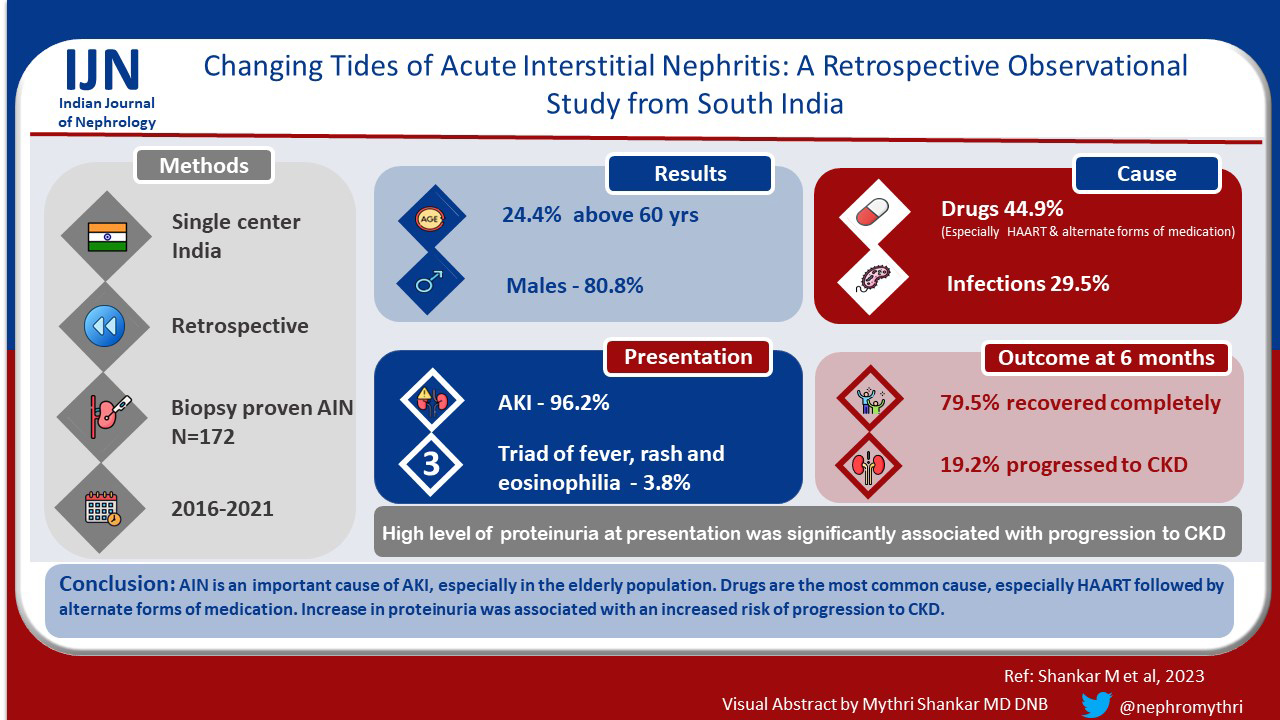
Among 6234 biopsies analyzed, there were 156 biopsy-proven AIN cases. The majority were in the 6th decade of life (24.4%) and males (80.8%). 50% of the patients had a history of drug intake, the most common being tenofovir (12.3%) followed by alternate forms of medications (10.3%). The majority (96.2%) presented with acute kidney injury (AKI). At the end of six months, 79.5% recovered completely, 19.2% progressed to chronic kidney disease. The presence of nephrotic range proteinuria at presentation was associated with progression to chronic kidney disease.
Conclusion:
AIN is an important cause of AKI, especially in the elderly population. Drugs are the most common cause, especially HAART follwed by alternate forms of medication. The presence of nephrotic range proteinuria was associated with increased risk of progression to chronic kidney disease.
Keywords
Acute interstitial nephritis
drugs
infections
Introduction
Inflammation of the interstitial kidney tissue was first described by Beamer A.[1] In the year 1898, Councilman described the “cellular and fluid exudation in the interstitial tissue” as a distinct entity called acute interstitial nephritis (AIN).[23] Most were children with scarlet fever and diphtheria. The kidneys were either infective, called infective AIN, or sterile, called reactive (allergic, non-infective) AIN.[23] Antibiotics, NSAIDs[678] proton pump inhibitors are the commonest drugs associated with AKI.[456789] Other known causes are autoimmune diseases such as systemic lupus erythematosus, sarcoidosis, IgG4 disease and Sjogren’s.[10]
The prognosis of AIN is variable. Infection and idiopathic AIN have higher rates of recovery.[8] The role of steroids in drug-induced AIN has been controversial.[111213] The cause for AIN in tropical countries can be entirely different from other parts of the world. We planned a retrospective study of all biopsy-proven cases of AIN from January 2016 to December 2021. The aim of the study was to investigate the causes of AIN, clinical presentations, outcomes and prognostic indicators.
Methods
A retrospective observational study was conducted in Institute of Nephrourology, Bengaluru. All (n = 6234) native kidney biopsies were reviewed from January 2016 to December 2021. All biopsy-proven AIN cases were included in the study.
AIN associated with systemic diseases (such as SLE, Sjogren’s syndrome, sarcoidosis, plasma cell dyscrasias), proliferative glomerulonephritis, and allograft biopsies were excluded. Cases with incomplete data were excluded.
As per the institutional treatment protocol, all biopsy-proven AIN cases were advised to discontinue the offending agent.
Steroids were considered in the following cases:
-
Non-recovery of AIN despite discontinuation of the offending agent such as discontinuation of the drug or treatment of infection (monitored over 3 to 7 days)
-
Severe cases at presentation such as the requirement of renal replacement therapy
Tapering doses of steroids (1 mg/kg body weight, maximum of 40 mg/day) for 6 to 8 weeks after ruling out active infection was considered in these cases.
Records were reviewed for demographic, causative agent, clinical, and laboratory data. The causative agent was determined by temporal association and the most probable cause by the treating nephrologists. The 6-month follow-up data of all patients was collected. Ethical clearance was taken from the institution’s Ethical Committee.
Outcomes were analyzed at the end of 6 months as complete recovery, partial recovery (progression to CKD), and death. Complete recovery was defined as improvement in s. creatinine levels to within 25% of baseline or s. creatinine of ≤1.4 mg/dl. Progression to CKD was defined as s. creatinine >1.4 mg/dl or >25% of baseline.[13]
Statistical methods
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on Mean ± SD (Min-Max). Significance is assessed at a 5% level of significance. Student t-test (two-tailed, independent) has been used to find the significance of study parameters on a continuous scale between two groups (Intergroup analysis) on metric parameters. Levene’s test for homogeneity of variance has been performed to assess the homogeneity of variance. Chi-square/Fisher Exact test has been used to find the significance of study parameters on a categorical scale between two or more groups.[1415]
Results
Among 6234 biopsies analyzed, 172 (2.74%) had AIN on kidney biopsy. 16 cases were excluded due to incomplete data. Finally, 156 biopsy-proven predominantly AIN cases were included in the study. The majority were elderly (>60 years)(24.4%) and males (80.8%). The mean age of presentation was 45.38 ± 17.74 years. Clinical presentation was variable. Fever was seen in 26.1% of patients and rash in 5.1%. 50% of the patients had a history of drug intake – the most common being tenofovir (12.3%) followed by alternate forms of medications (10.3%) and proton pump inhibitors (10.3%). 28.2% of the patients had a history of infections at presentation. The most common infection was HIV (11.1%), all these patients were detected to have HIV at the time of kidney biopsy before starting HARRT therapy [Table 1]. Among the comorbidities, 26.9% had diabetes mellitus and 44.9% were hypertensive. The majority (96.2%) presented with acute kidney injury (AKI), 2.6% with chronic kidney disease (CKD), and 1.3% with rapidly progressive renal failure (RPRF). 42.3% of patients required renal replacement therapy (RRT) at presentation.
| Cause | No. of Patients | % |
|---|---|---|
| Drug | 70 | 44.9 |
| Infection | 46 | 29.5 |
| Unknown | 22 | 14.1 |
| Rhabdomyolysis | 8 | 5.1 |
| Snake bite | 8 | 5.1 |
| Malignancy | 2 | 1.3 |
| Total | 156 | 100.0 |
41% had anemia at presentation. Eosinophilia was seen in 14.1% of patients. 38.5% had microscopic hematuria. 66.7% had WBCs in the urine but only 3.8% had eosinophils in the urine. The majority (60.3%) of the patients had <1g 24-hour proteinuria, 26.9% had 24-hour proteinuria between 1g and 3.5g (sub-nephrotic range) and nephrotic range proteinuria was seen in 12.8% of patients [Tables 2-4].
| Variables | Outcome | Total | P | |
|---|---|---|---|---|
| Recovery | Progression to CKD | |||
| Hemoglobin (g/dl) | ||||
| <10 | 56 (45.2%) | 8 (25%) | 64 (41%) | 0.343 |
| >10 | 68 (54.8%) | 24 (75%) | 92 (59%) | |
| Total | 124 (100%) | 32 (100%) | 156 (100%) | |
| eGFR (ml/min/1.73m2) | ||||
| ·≥60 | 2 (1.7%) | 0 (0%) | 2 (1.3%) | 0.794 |
| ·31–59 | 40 (33.9%) | 12 (37.5%) | 52 (34.7%) | |
| ·16–30 | 10 (8.5%) | 0 (0%) | 10 (6.7%) | |
| ·≤15 | 66 (55.9%) | 20 (62.5%) | 86 (57.3%) | |
| Total | 118 (100%) | 32 (100%) | 150 (100%) | |
Chi-squared test/Fisher’s exact test
| Variables | Outcome | Total | P | |
|---|---|---|---|---|
| Recovery | Progression to CKD | |||
| Age (years) | 45.73±17.85 | 44.06±17.85 | 45.38±17.75 | 0.741 |
| Hemoglobin (g/dl) | 10.1±2.32 | 10.16±2.51 | 10.11±2.35 | 0.919 |
| Eosinophilia (cells/mm3) | 774.75±325.54 | 795.33±411.63 | 780.36±328.88 | 0.932 |
| S. creatinine (mg/dl) | 6.33±4.68 | 6.21±5.35 | 6.31±4.79 | 0.925 |
| Variables | Outcome | Total | P | |
|---|---|---|---|---|
| Recovery | Progression to CKD | |||
| Urine microscopy- RBC per high power field | ||||
| < 3 | 64 (51.6%) | 10 (31.3%) | 74 (47.4%) | 0.240 |
| ≥ 3 | 60 (48.4%) | 22 (68.8%) | 82 (52.6%) | |
| Urine microscopy - Pus cells per high power field | ||||
| < 5 | 34 (27.41%) | 18 (56.25%) | 52 (33.33%) | 0.532 |
| ≥ 5 | 90 (72.5%) | 14 (43.75%) | 104 (66.7%) | |
| 24 -hour urine protein (grams) | ||||
| < 1 | 88 (71%) | 6 (18.8%) | 94 (60.3%) | < 0.001** |
| 1–3.5 | 30 (24.2%) | 12 (37.5%) | 42 (26.9%) | |
| > 3.5 | 6 (4.8%) | 14 (43.8%) | 20 (12.8%) | |
| Total | 124 (100%) | 32 (100%) | 156 (100%) | |
Chi-squared test/Fisher’s exact test
On kidney biopsy, pure AIN was seen in 60.2% of patients, predominantly AIN with acute tubular necrosis (ATN) was seen in 12.8%, predominantly AIN with focal segmental glomerulosclerosis (FSGS) (9%), predominantly AIN with pigment nephropathy (9%), predominantly AIN with minimal change disease (MCD) (3.8%) and AIN with necrotizing granuloma (1.3%). The causes of AIN were as follows: drug (44.9%), infection (29.5%), rhabdomyolysis (5.1%), snake bite (5.1%), and malignancy (1.3%). In 14.1% of the patients, the cause was not known - idiopathic AIN. More cases of drug-related AIN were associated with recovery (P = 0.05) compared to other causes [Table 5].
| Recovery | Progression to CKD | Total | P | |
|---|---|---|---|---|
| Drug intake | 56 (45.2%) | 24 (75%) | 80 (51.3%) | 0.057+ |
| Tenofovir | 18 (14.5%) | 2 (6.3%) | 20 (12.8%) | |
| Native medication (alternate forms of medicines) | 6 (4.8%) | 10 (31.3%) | 16 (10.3%) | |
| Proton pump inhibitor | 8 (6.5%) | 8 (25%) | 16 (10.3%) | |
| NSAID | 8 (6.5%) | 4 (12.5%) | 12 (7.7%) | |
| Cisplatin | 6 (4.8%) | 0 (0%) | 6 (3.8%) | |
| Rifampicin | 4 (3.2%) | 0 (0%) | 4 (2.6%) | |
| Carboplatin | 2 (1.6%) | 0 (0%) | 2 (1.3%) | |
| Meropenem | 2 (1.6%) | 0 (0%) | 2 (1.3%) | |
| Malignancy | 6 (4.8%) | 0 (0%) | 6 (3.8%) | 0.602 |
| Infection | 38 (30.6%) | 6 (18.8%) | 44 (28.2%) | 0.380 |
At the end of 6 months, 79.5% recovered completely, 19.2% progressed to CKD and two patients died due to cardiac events. The mean duration of patients requiring RRT at presentation to become dialysis independent was 13 ± 5 days. Only 3 patients (out of 66) remained dialysis-dependent at the end of 6 months; 2 of these had FSGS and the other patient had extensive (>50%) involvement of the interstitium. The presence of nephrotic range proteinuria at presentation was associated with progression to CKD. Usually, patients taking NSAIDs or alternate forms of medications had nephrotic syndrome with AIN [Tables 1-3].
Discussion
To the best of our knowledge, this is the largest study on AKI from India to date. AIN represents 1 to 3% of kidney biopsies according to some studies.[1617] This study had an incidence of 2.74% similar to the previous studies. This suggests that AIN is a common cause of AKI, but the actual incidence may be underestimated due to a variety of reasons. First, a biopsy is not done in all clinically suspected cases of AIN. Second, mild forms usually go undetected due to vague clinical complaints or AKI may be multifactorial.[18]
In this study, the most common cause was drug followed by infection and idiopathic. According to a review of three case series by Baker et al.,[19] more than two-thirds of AIN cases were drug-related. Drugs as the cause of AIN have been ever increasing since the beginning of the 19th century and infections have taken second place. A large and ever-increasing number of drugs have been implicated as the cause of AIN. Theoretically, any drug can cause AIN but the majority have been antibiotics followed by NSAIDs.[18] However, in this study the most common cause was tenofovir. This was followed by alternate forms of medications that are in rampant use in India. As most of the studies are from developed countries, alternate forms of medications causing the majority of AIN is not reported. Also, snake bite and rhabdomyolysis due to extensive hard labor in hot and humid conditions were causes of AIN in this study. These findings are representative of our population who are particularly from an agricultural background.
According to a series by Clarkson et al.,[11] fever was observed in 36%, rash in 22%, and eosinophilia (>500 cells per mm3) in 67%. This study observed fever in 26.1%, rash in 5.1% and eosinophilia in 14.1%. Only 3.8% had a triad of fever, rash and eosinophilia. This is consistent with data (<10%) from Clarkson et al.[11] and Gonzalez et al.[12] Microhematuria and leukocyturia were seen in 38.5% and 66.7% respectively, with eosinophils in urine seen in only 3.8% of patients. Urine eosinophils are not found to be useful in the diagnosis of AIN.[20] 90% to 100% presented with AKI and 40% required RRT at presentation[18] in some case series, similar to this study which showed 92.6% presented with AKI and 42.8% required RRT at presentation.
Large series[1112] showed that the majority had sub-nephrotic range proteinuria and only 2% had nephrotic range proteinuria, especially in NSAID associated AIN. This study also had similar results with the majority (87.2%) having sub-nephrotic range proteinuria and 12.8% having nephrotic range proteinuria seen especially in patients with NSAID and alternate medications intake related AIN.
In a study by Muriithi et al.,[13] 90% had complete or partial recovery at the end of 6 months and 5 to 10% progressed to CKD. In this study, 79.8% of patients recovered and 19.2% of patients progressed to CKD. The findings are similar, but the higher rate of progression could be attributed to the late presentation of the patients to tertiary care centers in developing countries. The mainstay of treatment for AIN has been the withdrawal of precipitating agents such as drugs and the treatment of infections. Nephrotic range proteinuria was significantly associated with progression to CKD in this study (P = 0.001). It was also noted that among the causes of AIN, drug-related AIN had better outcomes (P = 0.05) compared to other causes of AIN [Table 5] which is similar to the results of Clarkson et al.[11] and Muriithi et al.[13]
The strengths of the study include the large sample size, which is the highest to date as per our knowledge. This study is from a developing country in a tropical region, unlike previous studies which were mostly from developed countries. We could highlight the role of alternate forms of medication and snake bites as causes of AIN in this part of the world.
The limitation of the study is its retrospective design, and milder forms of AKI would have not undergone biopsy and might have been missed.
Conclusion
This study identifies AIN as one of the important causes of AKI especially in the elderly population. Drugs have been the most common cause, especially HAART and alternate forms of medication. Clinical presentation can be variable. Majority have complete recovery of kidney functions. Nephrotic range proteinuria is significantly associated with progression to CKD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Dr. Keshavamurthy, Director, Institute of Nephrourology, Dr. Umesha L, Professor and Ex-Head of department, Nephrology, Institute of Nephrourology, Faculty and residents of department of nephrology, Institute of Nephrourology, Nephropathologists Dr. Mahesha. V, Dr. Vinay. K. S, Dr. Vineeta Batra, Dr. Kiran.
References
- Renal failure and interstitial nephritis due to penicillin and methicillin. N Engl J Med. 1968;279:1245-52.
- [Google Scholar]
- The outcome of acute interstitial nephritis:risk factors for the transition from acute to chronic interstitial nephritis. Clin Nephrol. 2000;54:179-90.
- [Google Scholar]
- Proton pump inhibitors and the kidney: Critical review. Clin Nephrol. 2007;68:65-72.
- [Google Scholar]
- Diagnosis of IgG4- related tubulointerstitial nephritis. J Am Soc Nephrol. 2011;22:1343-52.
- [Google Scholar]
- Acute interstitial nephritis: Clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19:2778-83.
- [Google Scholar]
- Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73:940-6.
- [Google Scholar]
- Biopsy-proven acute interstitial nephritis, 1993-2011: A case series. Am J Kidney Dis. 2014;64:558-66.
- [Google Scholar]
- Fundamentals of Biostatistics. (5th ed). Pacific Grove, CA: Duxbury; 2000. p. :80-240.
- [Google Scholar]
- IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp;
- Allergic interstitial nephritis: Clinical features and pathogenesis. Q J Med. 1998;66:97-115.
- [Google Scholar]
- The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19:8-11.
- [Google Scholar]







