Translate this page into:
Clinical, Biochemical, and Histological Manifestations and Long-Term Outcomes of Renal Sarcoidosis - A Single Center Study
Corresponding author: Narayan Prasad, Department of Nephrology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow - 226014, Uttar Pradesh, India E-mail: narayan.nephro@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma H, Prasad N, Kaul A, Bhaduria D, Patel M, Behera M, et al. Clinical, Biochemical, and Histological Manifestations and Long-Term Outcomes of Renal Sarcoidosis - A Single Center Study. Indian J Nephrol. 2024;34:589-96. doi: 10.25259/ijn_222_23
Abstract
Background
Renal involvement in sarcoidosis is rare. We evaluated the pattern of renal involvement in sarcoidosis, its clinical course, renal histology, and response to treatment.
Materials and Methods
We retrospectively analyzed the data of all cases with sarcoidosis exhibiting renal involvement referred to our department between January 2010 and December 2021.
Results
A total of 33 patients (age: 50.6 ± 12.6 years, males: 57.6%) were analyzed. Common presenting symptoms were weight loss (81.8%; n = 27), fever (75.8%; n = 25), and vomiting (63.6%; n = 21). A total of 14 (42.4%) patients had granulomatous interstitial nephritis (GIN), 13 (39.4%) had isolated hypercalcemia, and six (18.2%) had GIN along with hypercalcemia. Renal biopsy was performed in 20 (60.6%) patients, and all showed GIN, with concomitant glomerular disease in four (12.1%) patients. Mean serum creatinine and 24-h urine protein at presentation were 4.3 ± 2.1 mg/dL and 2.5 ± 0.9 g/day, respectively. All patients received oral prednisolone 1 mg/kg/day with subsequent tapering, concomitantly with azathioprine. Mycophenolate mofetil was used in three (9.1%) patients who developed azathioprine-induced hepatoxicity. After a median follow-up of 24 months (8–120 months), mean serum creatinine and 24-h urine protein improved to 1.9 ± 1.5 mg/dL and 1.1 ± 0.6 g/day, respectively, (P = 0.005). On follow-up, two patients (6.1%) became dialysis-dependent, and three (9.1%) succumbed: one due to a cardiovascular event and two to sepsis and septic shock.
Conclusion
Granulomatous interstitial nephritis was the most common diagnosis in sarcoidosis patients with kidney failure. Early steroid treatment improves kidney function.
Keywords
Granulomatous interstitial nephritis
Hypercalcemia and acute kidney injury
Renal sarcoidosis
Introduction
Sarcoidosis is a chronic inflammatory multisystemic disorder characterized by non-caseating epithelioid granulomas involving mainly the cutaneous, pulmonary, and neurological systems.1 The indirect evidence of kidney involvement associated with hypercalcemia caused by the release of 1 alpha-hydroxylase from the non-caseating granuloma in sarcoid leads to hypercalcemia can cause acute kidney injury (AKI), and untreated patients may progress to chronic kidney disease.2
There are no definitive diagnostic criteria for sarcoidosis. The presence of hypercalcemia; the involvement of the organs, skin, lung, and neurological, in isolation or combination; and supportive histological manifestations aid in the diagnosis. It amalgamates clinical, histopathological, laboratory, and radiological findings. A high angiotensin-converting enzyme (ACE) level gives a clue to the disease.3 High ACE level correlates with the degree of granuloma; however, it may be non-specifically increased in other granulomatous and kidney diseases.4 Radiological findings are mainly due to typical or atypical involvement of lungs in sarcoidosis, sometimes lone radiology evidence for sarcoidosis.5 A non-caseating granuloma on histopathology points toward a likely sarcoid etiology.
Renal involvement due to sarcoidosis is uncommon, and only a few small and short-term follow-up studies have been published.6,7 The diagnosis requires a high index of suspicion. Its manifestation may range from granulomatous interstitial nephritis (GIN) to nephrocalcinosis, which might be the sole presentation even before the diagnosis of sarcoidosis.8 Knowledge of these syndromes related to sarcoidosis will prompt clinicians to keep sarcoidosis as differential in the above mentioned presentation. Renal involvement may not be the sole manifestation, and it may bring the patients to the nephrologist to manage renal failure. It is one of the most extensive series on histological findings on kidney biopsy to date. We aimed this study to analyze the clinical manifestations, kidney histology findings after a kidney biopsy, patterns of renal involvement, management, and long-term outcomes after treatment.
Materials and Methods
In this study, we retrieved the medical records of all sarcoidosis patients with renal involvement who have been diagnosed within the department or referred from other departments because of kidney involvement [Figure 1] during the study period (January 2010–December 2021). For all cases, the demographic and clinical information, such as age at presentation, gender, relevant past medical history, extrarenal involvement, serum creatinine (mg/dL), creatinine clearance, 24-h proteinuria, urinalysis, blood cell count, serum calcium (mg/dL) and serum ACE, and organ biopsy were recorded. Intact parathormone (iPTH), serum 25OH-vitamin D3 (25OHD3), and serum 1,25-dihydroxy-vitamin D3 values were collected. All patients received prednisolone 1 mg/kg of body weight and azathioprine 1.5 mg/kg of body weight. In case azathioprine-related side effects were encountered, mycophenolate mofetil was given. The ethics committee of the institute approved the study ethics approval number 2017-113-DM-IP-97, and waived off for the informed conset due to retrospective data retrieval for the study. The study was conducted per the guidelines of the Declaration of Helsinki for human studies.
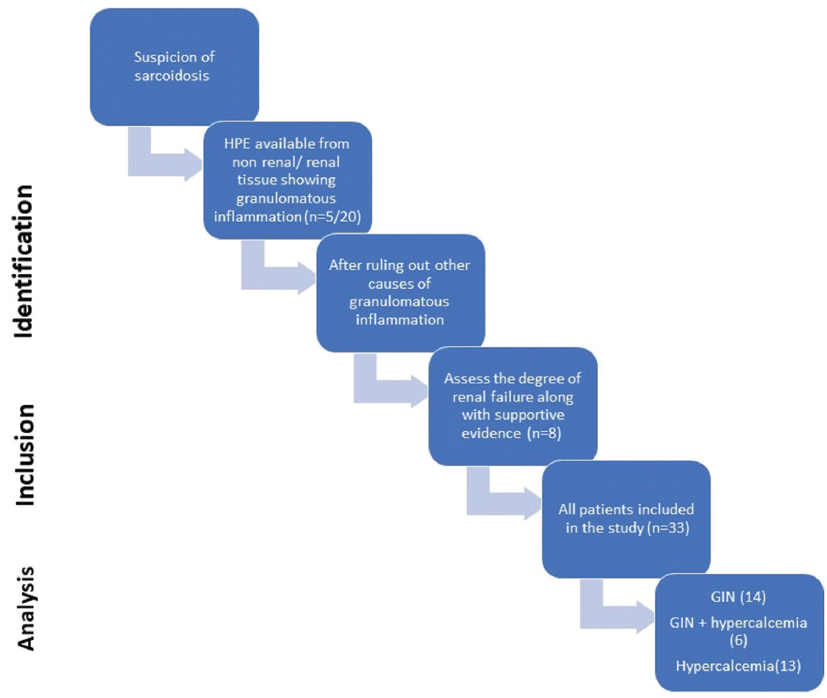
- Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) flowchart of the study. GIN: Granulomatous interstitial nephritis.
Hypercalcemia was defined as serum calcium greater than 2 standard deviations above the normal mean in a reference laboratory.9 Serum total calcium levels above 8.5 and 10.5 mg/dL were considered as hypercalcemia. Serum calcium levels above 10.5 mg/dL on two or more occasions were considered sustained hypercalcemia.10 Serum calcium levels between 2.62 and 3 mmol/L (10.6–12 mg/dL) were defined as mild, between 12 and 15 mg/dL as moderate, and above 3.7 mmol/L (15 mg/dL) as severe hypercalcemia. Hypercalcemia was managed with hydration, diuretics, intravenous bisphosphonate, and calcitonin as needed.
Native kidney biopsies were processed for light microscopy, immunohistochemistry, and electron microscopy if indicated. Two renal pathologists (MJ and VA) viewed all renal biopsies. The degree of interstitial fibrosis and tubular atrophy (IFTA) was classified as no IFTA (<10%), mild IFTA F1 (10%–25%), moderate IFTA F2 (25%–50%), and severe IFTA F3 (>50%). The presence of interstitial fibrosis, tubular atrophy, or both were considered for classifying the IFTA.
Patients were followed until their last visit to the outpatient clinics or the hospital until December 2022. The outcome and treatment for each patient was evaluated. Serum creatinine, mean estimated glomerular filtration rate (eGFR), and blood cell count were recorded at the last follow-up.
AKI was defined and staged per Kidney Disease Initiatives and Global Outcomes (KDIGO) criteria.5 CKD was classified based on the cause, GFR, and albuminuria categories mentioned in KDIGO.11,12 AKI was considered fully recovered if the patient achieved the baseline serum creatinine within 3 months. Partial recovery was defined as a reduction in serum creatinine but not reaching the baseline values. No change in the AKI stage over 3 months was defined as non-recovery.13 The need for dialysis, if any, was also recorded.
Statistical analysis
All data are expressed as mean values with standard deviation. The student’s t-test was used to compare the mean values between the groups. The categorical values were expressed in the form of percentages, and the comparison between the groups was performed using Fisher’s exact test or chi-square test as per the application required. A comparison between GIN and GIN with hypercalcemia was also conducted. The association of IFTA on histological findings with kidney outcomes was analyzed. Kaplan–Meier survival analysis was used to analyze the survival of the patients.
Results
The clinical characteristics of the patients are shown in Table 1. A total of 33 patients (mean age: 50.6 ± 12.6 years; males: 19,57.6%) were included. Twenty-eight patients (84.9%) presented first to the nephrology department, and five (15.1%) were referred from the gastroenterology (n = 1) and pulmonary medicine (n = 4) departments after having evidence of granulomas in the histopathological analysis of the respective organs and renal involvement. For patients referred from other departments, the mean period between diagnosis of sarcoidosis and kidney involvement was 3.2 ± 1.6 months. Of the 28 patients who presented to the nephrology department, only 10 had been diagnosed on the first visit (n = 10), 14 had been diagnosed on the follow-up visit (n = 14), and four during indoor admission (n = 4). Fourteen patients (42.4%) had hypertension, and nine (27.3%) had diabetes. Weight loss (81.8%) and fever (75.8%) were the common symptoms. Oliguria was observed in eight patients (24.2%), and five patients (15.1%) had asymptomatic kidney failure.
| Features | Total (N = 33) | GIN (N = 14) | Isolated Hypercalcemia (N = 13) | GIN and hypercalcemia (N = 6) | P value |
|---|---|---|---|---|---|
| Age (years) | 50.6 ± 12.6 | 44.2 ± 13.5 | 54.3 ± 9.9 | 57.6 ± 10.1 | 0.03 |
| Gender | 0.03 | ||||
| Male | 19 (57.6%) | 8 (57.1%) | 6 (46.1%) | 5 (83.3%) | |
| Female | 14 (42.4%) | 6 (42.9%) | 7 (53.9%) | 1 (16.7%) | |
| Symptoms | |||||
| Weight loss | 27 (81.8%) | 13 (92.9%) | 9 (69.2%) | 5 (83.3%) | 0.28 |
| Fever | 25 (75.8%) | 12 (85.7%) | 10 (76.9%) | 3 (50%) | 0.45 |
| Vomiting | 21 (63.6%) | 4 (28.6%) | 11 (84.6%) | 6 (100%) | 0.04 |
| Dry cough | 15 (45.4%) | 6 (42.9%) | 6 (46.2%) | 3 (50%) | 0.34 |
| Oliguria | 8 (24.2%) | 2 (14.3%) | 4 (30.7%) | 2 (33.3%) | 0.65 |
| Renal stone | 5 (15.1%) | 2 (6.1%) | 3 (9.1%) | 0 | 0.32 |
| Asymptomatic | 5 (15.1%) | 0 | 0 | 0 | 0.89 |
| ACE level | 0.64 | ||||
| Increased | 27 (81.8%) | 10 (71.4%) | 13 (100%) | 4 (66.7%) | |
| Normal | 4 (12.1%) | 2 (14.2%) | 0 | 2 (33.3%) | |
| Not done | 2 (6.1%) | 2 (14.2%) | 0 | 0 | |
| 1,25-dihydroxyvitamin D3 | 0.69 | ||||
| Not done | 14 (42.4%) | 4 (28.6%) | 7 (53.9%) | 3 (50%) | |
| Increased | 15 (45.5%) | 8 (57.1%) | 5 (38.5%) | 2 (33.3%) | |
| Normal | 4 (12.1%) | 2 (14.3%) | 1 (7.7%) | 1 (16.7%) | |
| Mean creatinine (mg/dl) at | |||||
| Admission | 4.3 ± 2.1 | 4.1 ± 2.1 | 3.4 ± 0.8 | 5.8 ± 2.4 | 0.04 |
| 1 month | 3.3 ± 1.6 | 3.1 ± 1.5 | 2.5 ± 0.7 | 4.6 ± 1.9 | 0.01 |
| 3 months | 2.5 ± 1.8 | 2.6 ± 2.1 | 1.6 ± 0.6 | 2.8 ± 1.6 | 0.19 |
| Last follow up | 1.9 ± 1.5 | 2.1 ± 1.7 | 1.3 ± 0.5 | 1.9 ± 1.3 | 0.25 |
| Mean 24-h urine protein (g/day) at | |||||
| Admission | 2.5 ± 0.9 | 2.6 ± 0.8 | 2.3 ± 0.8 | 2.6 ± 0.7 | 0.52 |
| 3 months | 1.1 ± 0.7 | 1.2 ± 0.6 | 0.9 ± 0.6 | 1.1 ± 0.6 | 0.57 |
| Hemoglobin (g/dL) | 9.1 ± 2.1 | 8.9 ± 2.1 | 10.1 ± 2.7 | 9.7 ± 2.3 | 0.35 |
| Mean serum calcium (mg/dL) | 9.8 ± 2.1 | 9.3 ± 1.3 | 11.4 ± 1.7 | 13.9 ± 3.4 | 0.04 |
| Dialysis requirement (at admission) | 3 (9.1%) | 1 (7.1%) | 0 | 2 (33.3%) | 0.33 |
| Treatment Prednisolone* | 33 (100%) | 14 (100%) | 13 (100%) | 6 (100%) | |
| Steroid sparing agent | |||||
| Azathioprine | 30 (90.9%) | 13 (92.8%) | 12 (92.3%) | 5 (83.3%) | 0.46 |
| Mycophenolate mofetil | 3 (9.1%) | 1 (7.1%) | 1 (7.7%) | 1 (16.7%) | |
| Renal recovery | 0.76 | ||||
| Yes | 30 (90.9%) | 12 (85.7%) | 13 (100%) | 5 (83.3%) | |
| No | 3 (9.1%) | 2 (14.3%) | 0 | 1 (16.7%) | |
| Residual renal damage (at the end of 3 months) | 0.43 | ||||
| Yes | 18 (54.5%) | 8 (57.1%) | 6 (46.1%) | 4 (66.6%) | |
| No | 15 (45.4%) | 6 (42.9%) | 7 (53.9%) | 2 (33.4%) | |
| Dialysis dependency (at last follow-up) | 2 (6.1%) | 1 (7.1%) | 0 | 1 (16.7%) | 0.24 |
| Patient status | |||||
| Live | 30 (90.9%) | 13 (92.8%) | 11 (84.6%) | 6 (100%) | 0.52 |
| Death | 3 (9.1%) | 1 (7.2%) | 2 (15.4%) | 0 |
*Prednisolone, at the dose of 1 mg/kg, was given to all patients, with tapering started at 1 month of therapy. ACE: Angiotensin-converting enzyme.
Hypercalcemia was observed in 19 (57.6%) patients with renal failure. The mean corrected serum calcium level was 13.60 ± 2.33 mg/dL. The serum 25 (OH)D3 level was low (<30 ng/mL) in all patients, while the 1,25 DihydroxyD3 level was raised in 15 of 33 patients irrespective of calcium levels, normal in four of 33 patients, and could not be done for 14 patients. In patients with hypercalcemia (n = 19), iPTH levels were lower than the normal range in all (iPTH < 15 pg/mL). Renal stone was observed in five (15.1%) patients, of which three (9.1%) patients had concomitant hypercalcemia and two (6.1%) patients did not have hypercalcemia.
Six (18.2%) patients had both GIN and hypercalcemia, of which two had tubulointerstitial calcification, and 13 (39.3%) had isolated hypercalcemia. Patients in the hypercalcemia group were older and had a dominant vomiting symptom, attributed to their high calcium levels. Mean creatinine was higher in patients with GIN and hypercalcemia at the time of presentation and gradually decreased with treatment, showing a significant difference at 1 month only [Table 1].
Organ involvement and staging as per Scadding criteria
The organ involvement and staging were performed as per Scadding criteria.8 Lung involvement was observed in 100% of patients. As per Scadding criteria, stage 1 and stage 2 were observed in 14 patients (42.4%) each, stage 3 in three (9.1%) patients, and stage 4 in two (6.1%) patients All cases were confirmed with high-resolution computed tomography (HRCT) of the thorax.14 Four patients (12.1%) had predominantly respiratory symptoms and presented to the pulmonary medicine department. Radio imaging showed hilar and parenchymal involvement, and histopathological examination of lymph nodes revealed granulomas. On further evaluation, they were found to have hypercalcemia and renal involvement, hence referred to our department for further evaluation. Similarly, during the workup of one patient (3.1%) for refractory vomiting, hepatomegaly, deranged liver function test, and renal function test, characteristic granulomas of sarcoid were seen on liver biopsy, and the patient was referred to the nephrology department.
Pattern of renal involvement
All patients presented to the department had low eGFR at diagnosis. The mean serum creatinine level at presentation was 4.3 ± 2.1 mg/dL, eGFR was 16 ± 6 mL/min, and mean creatinine clearance was 19 ± 12 mL/min. The mean 24-h urine protein was 2.5 ± 0.9 g/day. 30 patients (90.9%) had normal kidney size and AKI, with a mean creatinine at admission of 3.8 ± 1.8 mg/dL and were categorized as AKI. The remaining three patients (9.1%) had small-sized kidneys on ultrasound, with a mean creatinine at admission of 4.8 ± 2.1 mg/dL, and they were labeled as acute on chronic kidney disease (CKD). Most patients (n = 27; 81.8%) had anemia (mean: Hb 9.1 ± 2.1 g/dL). Levels of ACE were elevated in 27 (81.8%), normal in four (12.1%), and not done in two patients (6.1%). At admission, three (9.1%) patients required renal replacement therapy.
Histological manifestations
Kidney biopsy was performed in 20 patients (60.6%). A representative microphotograph of sarcoid granuloma is shown in Figure 2. All showed non-caseating granulomatous interstitial inflammation, and some had the features of concomitant glomerulonephritis such as diffuse global glomerular sclerosis (DGGS) with IgA deposits in two biopsies (6.1%) and minimal change disease (MCD) and membranoproliferative glomerulonephritis (MPGN) pattern in one patient each. Tubulointerstitial calcification was seen in two patients (6.1%), and both had hypercalcemia and hypercalciuria. Overt nephrocalcinosis was not seen in any of the cases. Thirteen patients with hypercalcemia were not biopsied, either because the kidney was non-amenable to biopsy or consent was not given. Involvement of other organs was assessed, and after ruling out other causes of hypercalcemia, a diagnosis of sarcoidosis was made.
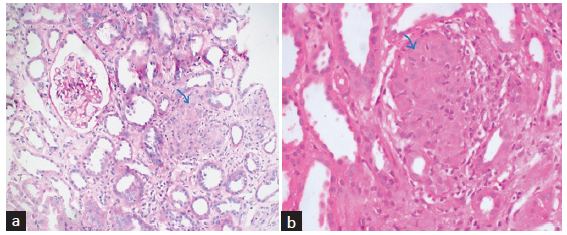
- (a) Section from renal cortex shows circumscribed non-necrotizing granuloma comprising epithelioid histiocytes and lymphocytes (blue arrow). PAS stain ×200 original magnification. (b) Section from the renal cortex shows circumscribed non-necrotizing granuloma composed of epithelioid histiocytes and lymphocytes (blue arrow) in another patient. H&E stain ×400 original magnification.
On analyzing the histopathological findings of patients with GIN and those with GIN and hypercalcemia [Table 2], we observed that both groups had similar IFTA grading and grading of interstitial inflammation. However, the GIN group of patients alone had significantly more globally sclerosed glomeruli. The serum creatinine decline in different IFTA groups of patients revealed that no IFTA had achieved normal serum creatinine value at the end of the 3-month follow-up. In contrast, the patients with mild, moderate, and severe IFTA had shown a trend of decline, but the renal failure persisted at 3 months and at the end of the follow-up [Table 3].
| GIN (N = 14) | GIN and Hypercalcemia (N = 6) | P value | |
|---|---|---|---|
| No. of glomeruli (mean) | 11.4 ± 5.5 | 12.8 ± 4.1 | 0.34 |
| Globally sclerosed glomeruli (percentage) | 35.1 ± 12.2 | 9.8 ± 5.6 | 0.03 |
| Tubulointerstitial fibrosis and atrophy (IFTA) | |||
| No IFTA | 4 (28.6%) | 2 (33.4%) | 0.27 |
| Mild (F1) | 4 (28.6%) | 4 (66.6%) | |
| Moderate (F2) | 4 (28.6%) | 0 | |
| Severe (F3) | 2 (14.3%) | 0 | |
| Interstitial inflammation | |||
| No inflammation | 4 (28.6%) | 2 (33.3%) | 0.65 |
| Mild (I1) | 3 (21.4%) | 3 (50%) | |
| Moderate (I2) | 3 (21.4%) | 0 | |
| Severe (I3) | 4 (28.6%) | 1 (16.7%) | |
| IF findings | Non-specific C3 deposits | No deposits seen |
GIN: Granulomatous interstitial nephritis; F1: 10-25%, F2: 25-50%, F3: >50% interstitial fibrosis and tubular atrophy; I1<25%, I2 25-50%, and I3 > 50% interstitial inflammation; C3: Complement, IF: Immunofluorescence
| No IFTA (n = 6) | Mild IFTA (F1) (n = 8) | Moderate IFTA (F2) (n = 4) | Severe IFTA (F3) (n = 2) | P value | |
|---|---|---|---|---|---|
| Mean serum creatinine at admission (mg/dL) | 2.9 ± 1.0 | 4.8 ± 2.4 | 6.0 ± 2.7 | 6.7 ± 1.5 | 0.09 |
| Mean serum creatinine at 1 month (mg/dL) | 2.2 ± 0.9 | 3.7 ± 1.9 | 4.2 ± 1.2 | 5.4 ± 1.1 | 0.07 |
| Mean serum creatinine at 3 months (mg/dL) | 1.3 ± 0.5 | 2.3 ± 1.4 | 3.6 ± 1.3 | 4.2 ± 1.4 | 0.04 |
| Mean serum creatinine at follow-up (mg/dL) | 1.0 ± 0.2 | 1.7 ± 1.2 | 3.0 ± 1.8 | 3.2 ± 1.1 | 0.01 |
| 24-h urine protein at admission (g/day) | 2.5 ± 1.1 | 2.3 ± 0.5 | 3.2 ± 0.4 | 3 ± 0.3 | 0.17 |
| 24-h urine protein at follow-up (g/day) | 0.7 ± 0.3 | 1.0 ± 0.5 | 1.7 ± 0.5 | 2.1 ± 0.3 | 0.001 |
IFTA: Interstitial fibrosis and tubular atrophy
The degree of proteinuria was higher in moderate and severe IFTA groups than in mild and no IFTA groups of patients. However, the decline in proteinuria was also observed in all groups of patients with treatment. The degree of interstitial fibrosis and tubular atrophy affects the degree of renal failure in terms of serum creatinine value and 24-h urine protein at admission, 1 month, and 3 months of the disease and follow-up [Table 3, Figure 3]. However, the degree of inflammation does not affect the renal failure.
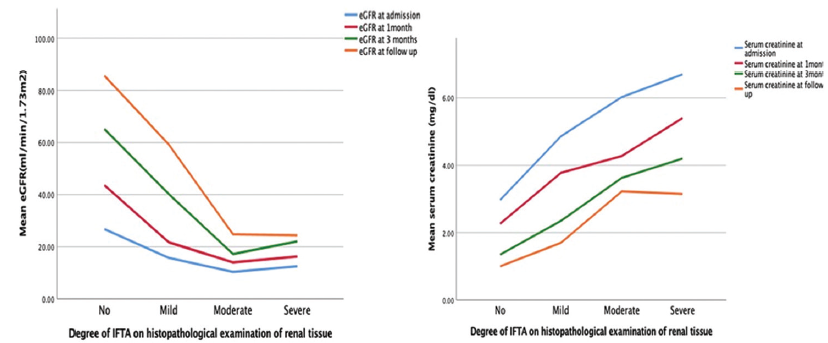
- Effect of the degree of IFTA on serum creatinine and eGFR (P value across all categories of IFTA for serum creatinine and eGFR at 3-month follow-up. IFTA: Interstitial fibrosis and tubular atrophy, eGFR: Estimated glomerular filtration rate.
Treatment and outcomes
Prednisolone 1 mg/kg and azathioprine were given to all patients. Tapering of prednisolone was started after 1 month of the therapy with 20% every 2 weeks and maintained on 5–10 mg dose depending on clinical response to maintain remission. Azathioprine was started concomitantly with prednisolone. Three patients (9.1%) were switched to MMF from azathioprine after noticing azathioprine-induced hepatotoxicity. The mean duration of the treatment was 34.3 ± 16.7 months. In all patients, hypercalcemia was managed initially with hydration and diuretics, while two patients with GIN and hypercalcemia and one patient with GIN had volume overload and received dialysis. All three patients became dialysis-independent after treatment and had partially recovered renal function. Serum creatinine showed a declining trend with steroid therapy. Similarly, proteinuria also recovered with treatment [Figure 4]. Hypercalcemia settled with hydration and subsequent steroid therapy in all patients. The stopping decision of maintenance immunosuppression was based on response to the treatment in achieving remission, and a low dose of prednisolone (5–10 mg) was continued minimum for 6 months after remission as per our protocol.
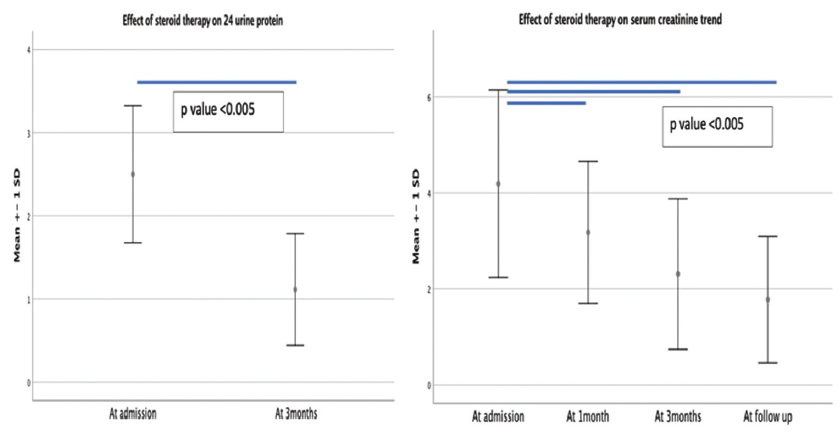
- Effect of immunosuppressive therapy on 24-h urine protein and serum creatinine value.
After therapy, renal function improved, and 30 (90.9%) patients had recovered renal function. Three patients (9.1%) did not show recovery; neither was there a change of stage nor did they achieve baseline creatinine. At the end of 3 months, residual renal damage in the form of proteinuria (>500 mg/day) and raised creatinine from baseline was present in 15 patients (55.6%). At the end of follow-up, two out of three patients who required renal replacement therapy initially became dialysis-dependent.
Patients were followed up for a median duration of 24 months (8–120 months). During follow-up, three patients (9.1%) died: one to a cardiovascular event and two to sepsis and septic shock. The estimated patient survival on Kaplan–Meier survival analysis at 12, 18, 36, and 120 months was 96%, 93.5%, 82.6%, and 82.6%, respectively [Figure 5].
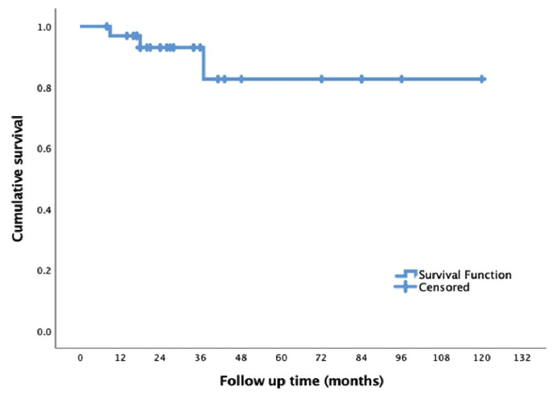
- Kaplan–Meier survival analysis showing patient survival on follow-up.
Discussion
This study highlights the varied forms of renal involvement in sarcoidosis, their clinical presentation, histopathological findings, management, and outcomes of the patients. Renal involvement in sarcoidosis is uncommon, occurring in 0.7%–4.3% of sarcoidosis patients.15 A high index of clinical suspicion is required for diagnosing renal sarcoidosis, which is evident from our findings as 15% of patients from other specialties had incidental findings of renal involvement due to sarcoidosis and were referred to the concerned clinician accordingly.
In our study, 20 patients had tissue diagnosis of GIN, out of which six patients had concomitant hypercalcemia while the remaining 13 patients underwent workup for PTH independent hypercalcemia where PTH levels were low with high calcium levels, indicating suppressed PTH secretion due to high calcium from any other sources. The diagnosis of sarcoidosis was made based on other radiological findings, increased vitamin D activity, and excluding the malignant conditions causing hypercalcemia. Thirteen patients with hypercalcemia were not biopsied. A diagnosis of sarcoidosis was made based on other organ involvement and after ruling out other causes of hypercalcemia. Our study focused on three patterns of renal involvement in sarcoidosis. GIN was the most common pattern seen in 42.4% of patients, followed by isolated hypercalcemia in 39.3%, and 18.3% had concomitant GIN and hypercalcemia. It is consistent with most studies where GIN followed by hypercalcemia was mentioned as the most common finding in sarcoidosis patients with renal involvement.7,16-18 Correia et al. reported that being a disease of adulthood, most symptoms manifest between 20 and 39 years of age.8 On analyzing three groups, it was deduced that our cohort had patients with a mean age of 50.6 ± 12.6 years. It was evident that patients with simultaneous presentation of GIN with hypercalcemia were elderly.
Our study illustrated the histopathological findings of 20 patients. GIN was the most common histopathological finding. It was studied for the first time in a series of sarcoidosis that the degree of IFTA affected the recovery of renal function. We also observed that glomerular involvement was uncommon, as observed in other studies in 4%–26% of cases of sarcoidosis. Though the exact mechanism is unknown, cytokines produced by sarcoid granulomas, circulating immune complexes, and hypergammaglobulinemia may contribute to glomerular injury in sarcoidosis. IgAN and membranous nephropathy are the most common glomerular lesions described in the series, while our series reports three lesions, namely IgAN, MCD, and MPGN, which were steroid responsive, unlike described glomerular lesions with sarcoidosis in other series.19
Other findings include calcifications, which may correspond to hypercalcemia and hypercalciuria. Along with other described lesions in sarcoidosis, overt nephrocalcinosis was seen in 10%–15% of cases in different series.16,20 However, in our series, none of the patients had overt nephrocalcinosis; only two patients had renal stones, and a biopsy revealed tubulointerstitial calcification in two patients attributed to their high calcium levels and hypercalciuria. In our series, six patients (18.2%) who underwent biopsy had hypercalcemia. They might have had double hits to the renal tissue presenting with a higher degree of renal failure, IFTA, because of persistent hypercalcemia for a longer duration. The biopsy was done in these patients with the suspicion of a higher degree of tissue damage. However, their renal and patient outcomes with and without hypercalcemia were similar. Like other groups, they responded well to steroids and immunosuppressive and recovered renal function without any relapse during the follow-up period. Hypercalcemia is the predominant manifestation in our series, particularly in the elderly. Hypercalcemia may be common in CKD patients due to drug therapy, calcium-based phosphate binders, calcitriol therapy, and tertiary hyperparathyroidism.21 The role of ACE level in diagnosing sarcoidosis with renal failure has been controversial, and its level as supportive evidence helped us diagnose patients with normal renal function. The mean value at the time of diagnosis was 67.6 ± 12.4 U/dL; however, it was not followed up after the completion of treatment.22
Standard protocols of treatment and extensive literature on response are sparse. However, systematic reviews suggested that roughly 90% of patients with GIN or hypercalcemia responded well to immune suppression.5 In our study too, all patients received corticosteroids and azathioprine; however, three patients who switched to MMF from azathioprine due to hepatoxicity showed satisfactory responses. The immunosuppression dose was modified based on clinical and biochemical responses during the treatment course. We did not use methotrexate in patients with renal sarcoid, and monoclonal antibodies were also not used, mainly because of fear of activation of latent tuberculosis, which is highly prevalent in our country. Despite the prolonged duration of the disease and higher degree of renal failure and IFTA on histology, patients showed good response in terms of renal recovery. However, with a median duration of 24 months (8–120 months) follow-up, our cohort showed residual renal damage, which may be due to the late detection and delayed institution of treatment for renal involvement in sarcoidosis. The partial recovery may be due to underlying irreversible changes at the tissue level in terms of IFTA and glomerulosclerosis. In contrast to the previous studies, all patient in our study had low GFR at the time of presentation (mean GFR: 16.5 ± 6.5 mL/min/1.73 m2) and still showed a good response. The time of detection of renal failure to the treatment may be crucial and needs to be reduced to prevent residual renal damage.7
Renal sarcoidosis is uncommon and requires a high index of suspicion for diagnosis. Subtle and non-specific symptoms should prompt nephrologists to look for sarcoidosis, particularly with multi-organ involvement. GIN is the common histological manifestation, and hypercalcemia is the most common biochemical abnormality. The presence of chronicity and IFTA indicates slow ongoing injury, and treatment with corticosteroids shows response in terms of renal recovery despite chronicity. The long-term outcomes are satisfactory with corticosteroid therapy.
Conflicts of interest
There are no conflicts of interest.
References
- Sarcoidosis: Causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sarcoidosis: The neph- rologist’s perspective. Am J Kidney Dis. 2006;48:856-70.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic utility of angiotensin-converting enzyme in sarcoidosis: A population-based study. Lung. 2016;194:91-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum ACE level in sarcoidosis patients with typical and atypical HRCT manifestation. Pol J Radiol. 2016;81:458-61.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and detection of sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal sarcoidosis: Epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2015;31:306-15.
- [PubMed] [Google Scholar]
- Renal sarcoidosis: Clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore). 2009;88:98-106.
- [CrossRef] [PubMed] [Google Scholar]
- Renal manifestations of sarcoidosis: From accurate diagnosis to specific treatment. Int Braz J Urol Off J Braz Soc Urol. 2020;46:15-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Approach to hypercalcemia. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. Available from http://www.ncbi.nlm.nih.gov/books/NBK279129/ [Last accessed on 2022 Jan 28]
- [Google Scholar]
- The changing profile of hypercalcemia in a tertiary care setting in North India: An 18-month retrospective study. Clin Cases Miner Bone Metab. 2017;14:131-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-84.
- [CrossRef] [PubMed] [Google Scholar]
- CKD Evaluation and Management – KDIGO. Available from: https://kdigo.org/guidelines/ckd-evaluation-and-management/. [Last accessed on 2022 Dec 24].
- Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Imaging of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:45-53.
- [CrossRef] [PubMed] [Google Scholar]
- Sarcoidosis with renal involvement. Postgrad Med J. 1978;54:528-32.
- [CrossRef] [PubMed] [Google Scholar]
- Sarcoid tub- ulo-interstitial nephritis: Long-term outcome and response to corticosteroid therapy. Kidney Int. 2006;70:165-9.
- [CrossRef] [PubMed] [Google Scholar]
- Renal sarcoidosis: Clinical, laboratory, and histologic presentation and out- come in 47 patients. Medicine. 2009;88:98-106.
- [CrossRef] [PubMed] [Google Scholar]
- Sarcoidosis in native and transplanted kidneys: Incidence, pathologic findings, and clinical course. PLoS One. 2014;9:e110778.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinicopathological study of glomerular diseases associated with sarcoidosis: A multi- centre study. Orphanet J Rare Dis. 2013;8:65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Renal sarcoidosis: Epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2015;31:306-15.
- [PubMed] [Google Scholar]
- Secondary and tertiary hyperparathyroidism. J Clin Densitom Off J Int Soc Clin Densitom. 2013;16:64-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reconsideration of the cut-off value of angiotensin-converting enzyme for screening of sarcoidosis in Japanese patients. J Cardiol. 2019;74:507-11.
- [CrossRef] [PubMed] [Google Scholar]







